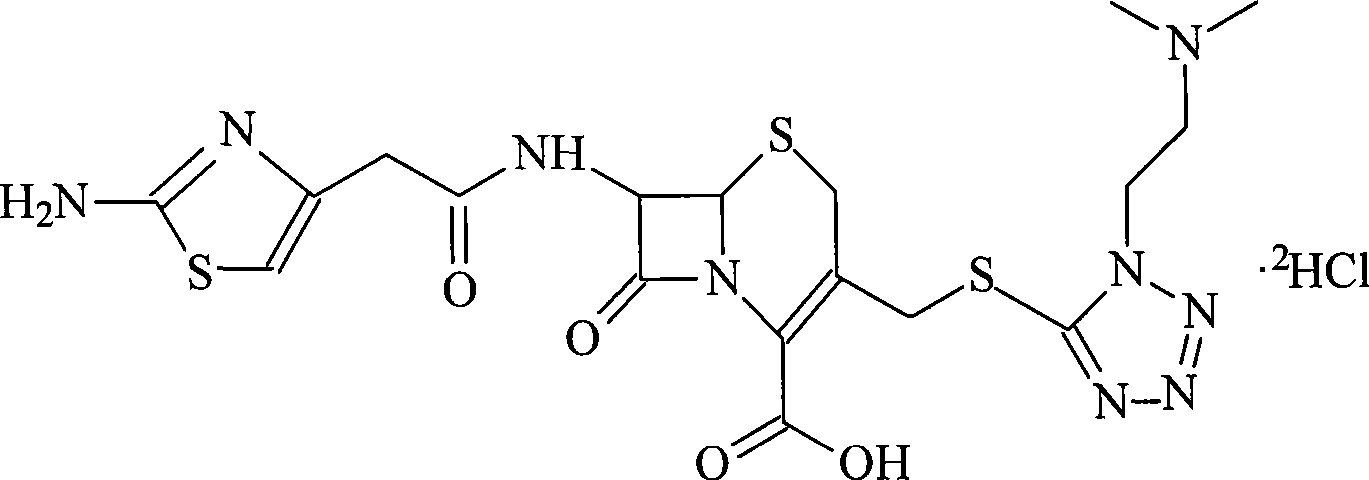
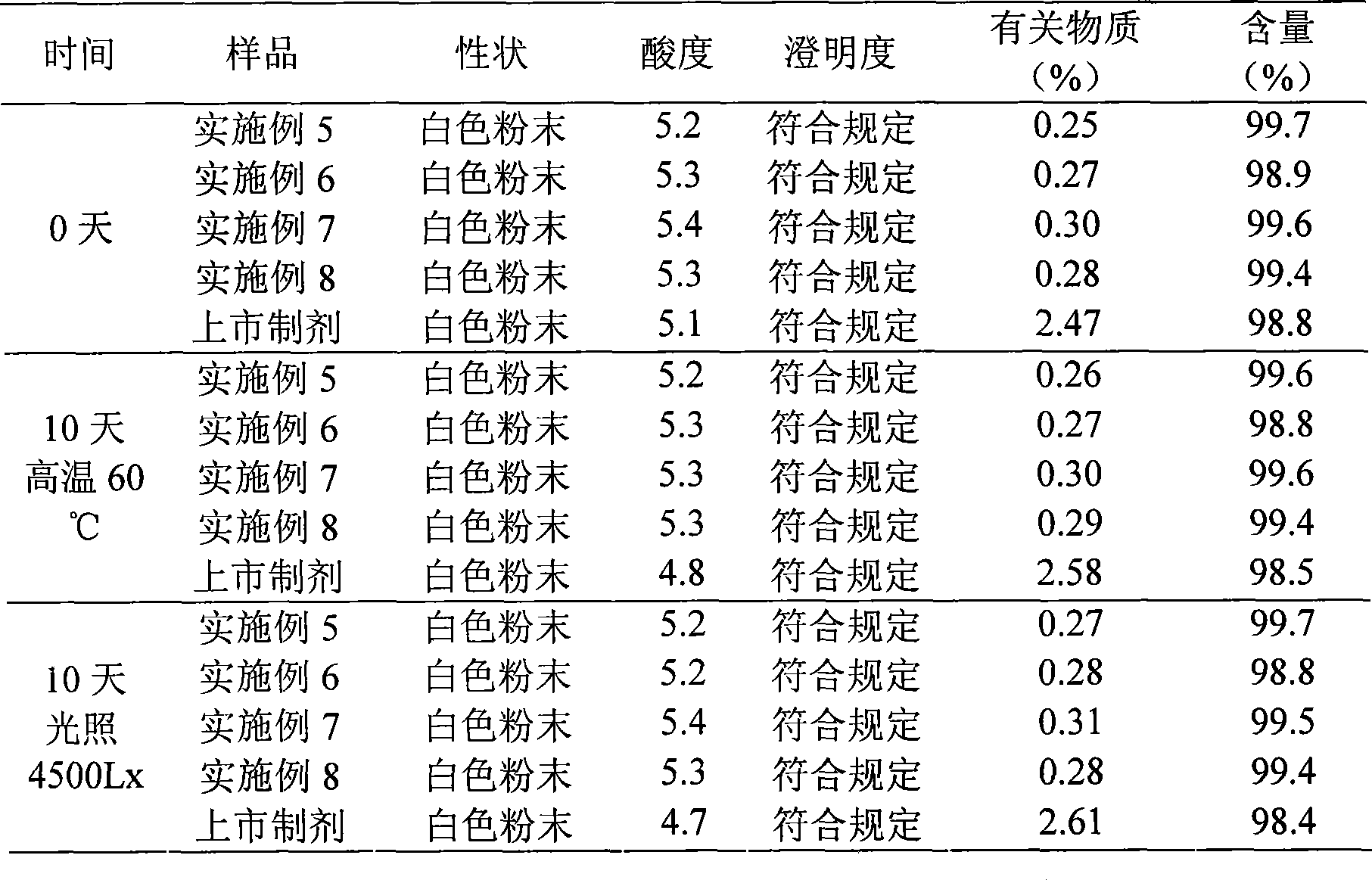
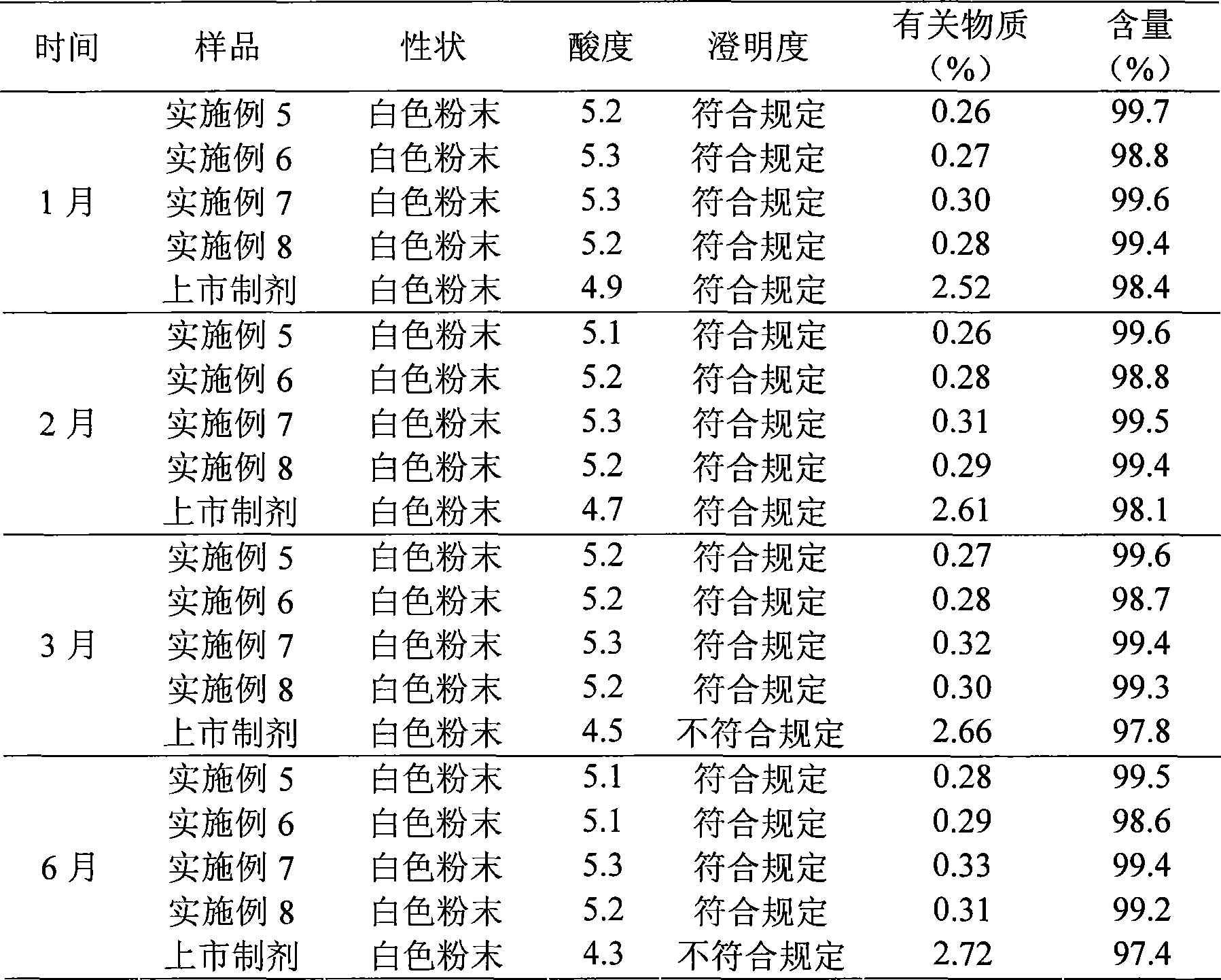
Cefotiam salt compound and pharmaceutical composition made therefrom
A technology of cefotiam and cefotiam hydrochloride, which is applied in the field of cefotiam hydrochloride, can solve the problems of high cefotiam polymer content, reduced drug active ingredient content, increased polymer impurity content, etc. The effect of low content, long validity period and high crystal purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Crude cefotiam hydrochloride (produced by Jiangsu Jiushoutang Biological Products Co., Ltd., batch number 20080611), the content of cefotiam recorded by high performance liquid chromatography is 76.8%, and the content of high molecular polymer measured by molecular exclusion chromatography is 6.3% .
[0036] 1). Add 100 g of the crude product of cefotiam hydrochloride into 300 ml of water, stir to dissolve it, and obtain an aqueous solution of cefotiam hydrochloride;
[0037] 2). Cool the aqueous solution to 0°C with cold water, add 2 mol / L sodium hydroxide solution dropwise to the cefotiam hydrochloride aqueous solution under stirring conditions, adjust the pH to 2.0, and white precipitates begin to precipitate;
[0038] 3). Remove the precipitate by filtration to obtain the filtrate; then, cool the obtained filtrate to 0°C with cold water, continue to add dropwise 2mol / L sodium hydroxide solution under stirring, adjust the pH to 3.5, and crystals of cefotiam hydrochlo...
Embodiment 2
[0040] Crude cefotiam (produced by Jiangsu Jiushoutang Biological Products Co., Ltd., batch number 20080523), the content of cefotiam measured by high performance liquid chromatography is 90.1%, and the content of high molecular polymer determined by size exclusion chromatography is 8.6%.
[0041]1). Add 100 g of the above-mentioned cefotiam crude product into 500 ml of water, add hydrochloric acid solution dropwise while stirring to dissolve it, and obtain cefotiam hydrochloride aqueous solution;
[0042] 2). Cool the aqueous solution to 5°C with cold water, add 20% ammonia water dropwise under stirring conditions, adjust the pH to 3.0, and white precipitates begin to precipitate;
[0043] 3). Remove the precipitate by filtration to obtain the filtrate, then add 1.5g of activated carbon, stir and decolorize for 30 minutes, filter and decarburize to obtain the filtrate, cool the filtrate to 5°C, continue to add 20% ammonia water dropwise under stirring, and adjust the pH to 5.0...
Embodiment 3
[0045] Cefotiam hydrochloride powder injection (produced by Shanghai Xinfeng Pharmaceutical Co., Ltd., batch number 20080912), the content of cefotiam recorded by high performance liquid chromatography is 78.3%, and the content of high molecular polymer measured by molecular exclusion chromatography is 5.9%. .
[0046] 1). Add 100 g of the crude product of cefotiam hydrochloride powder injection into 400 ml of water, stir to dissolve it, and obtain an aqueous solution of cefotiam hydrochloride;
[0047] 2). Cool the aqueous solution to about 15°C with cold water, add 1.5 mol / L sodium acetate solution dropwise to the cefotiam hydrochloride aqueous solution under stirring conditions, adjust the pH to 2.7, and white precipitates begin to precipitate;
[0048] 3) Filtration to remove the precipitate to obtain the filtrate; then the obtained filtrate was cooled to about 8°C with cold water, and the sodium acetate solution of 1.5mol / L was continued to be added dropwise under stirrin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com