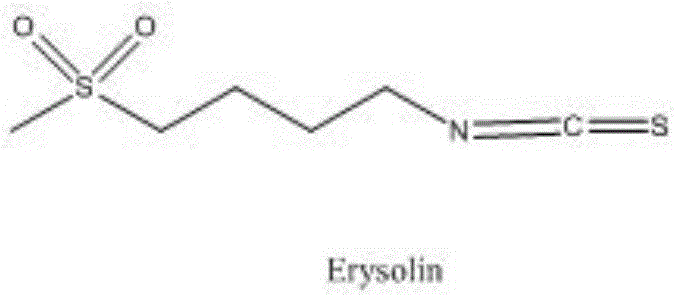
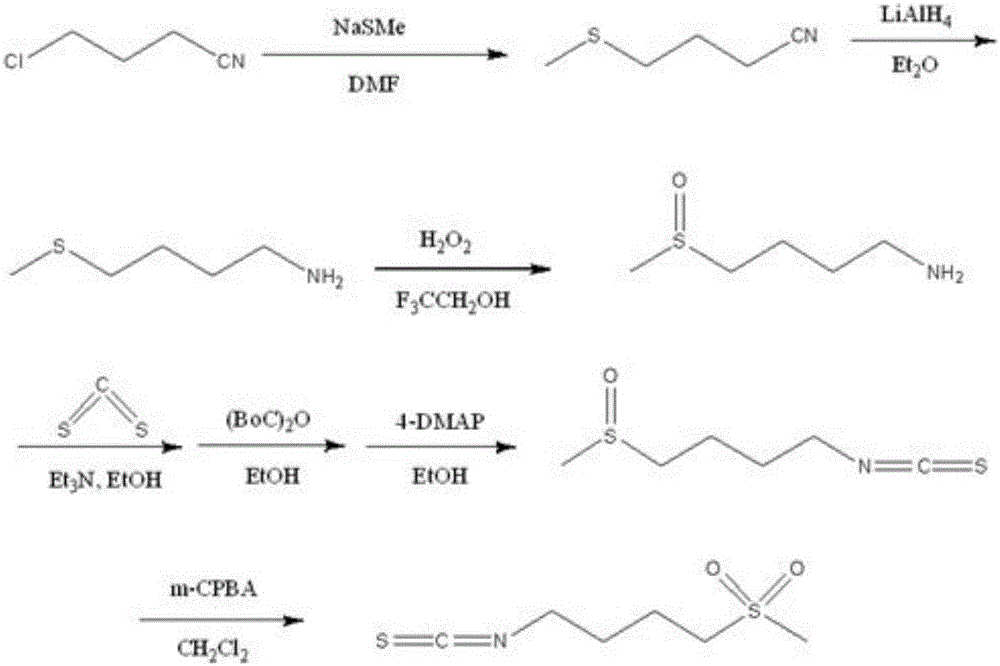
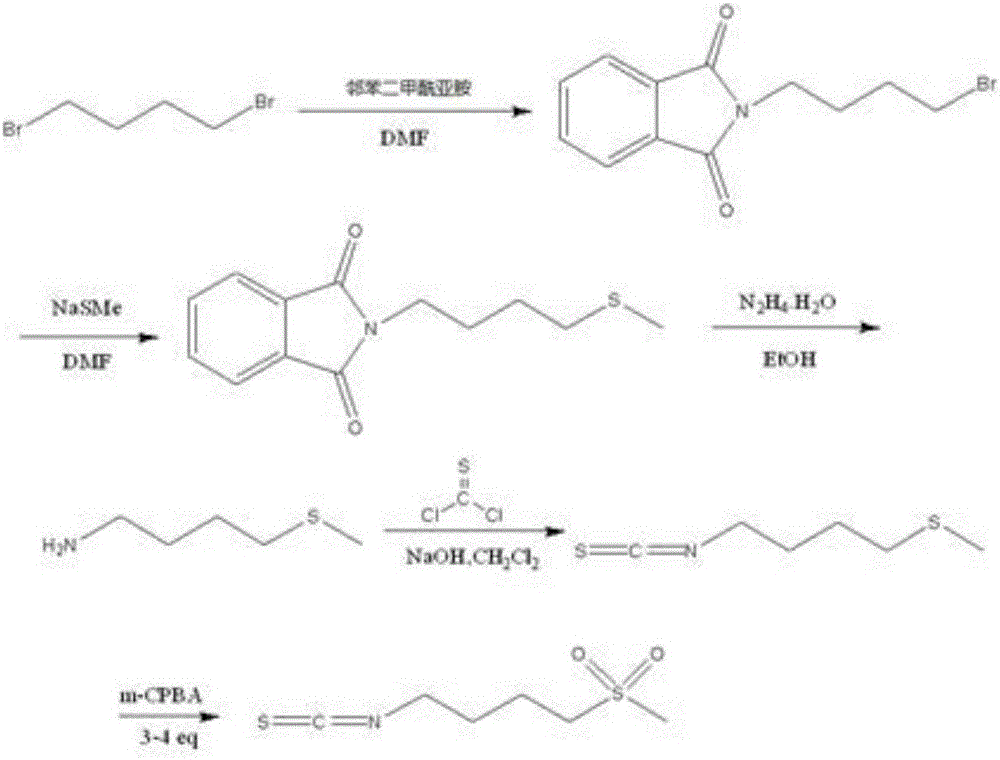
Synthesis method of 4-methanesulfonylbutyl isothiocyanate
A technology of methanesulfonylbutyl isothiocyanate and a synthesis method, which is applied in the field of chemical synthesis of 4-methanesulfonylbutyl isothiocyanate, can solve problems such as high cost of anhydrous sodium methanethiolate, and achieve cost reduction, The effect of reduced overall yield and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Dissolve 3.53g (20.6mmol) of 1-bromo-4-chlorobutane in 50ml of anhydrous DMF, add to a 250ml round-bottomed flask, stir in an ice bath at 0°C, and slowly drop in the solution containing 3.03g (21.63mmol, 1.05 times the amount) of 50% sodium methyl mercaptan in absolute ethanol was naturally warmed to room temperature and left overnight. The total reaction time was 20h. Stirring was stopped, and the ethanol was removed by rotary evaporation at low temperature in vacuum, extracted three times continuously with n-hexane, washed with saturated sodium chloride successively, dried the extract with anhydrous sodium sulfate, and filtered off the insoluble matter. The n-hexane was removed by low-temperature rotary evaporation under vacuum to obtain 2.30 g of a colorless oily substance with a strong mustard smell. The intermediate content was determined to be about 78.8% by GC-MS, and the yield of intermediate I was about 63.3%. Mass spectral data (GC-MS, EI): 138.1, 103.1, 9...
Embodiment 2
[0055] (1) Dissolve 3.53g (20.6mmol) of 1-bromo-4-chlorobutane in 50ml of absolute ethanol, add to a 250ml round-bottomed flask, stir in an ice bath at 0°C, and slowly drop in the solution containing 3. 11 g (22.2 mmol, 1.08 times the amount) of 50% sodium methyl mercaptide in absolute ethanol was naturally warmed to room temperature and left overnight. The total reaction time was 26 hours. Stirring was stopped, and the ethanol was removed by rotary evaporation at low temperature in vacuum, extracted three times continuously with n-hexane, washed with saturated sodium chloride successively, dried the extract with anhydrous sodium sulfate, and filtered off the insoluble matter. The n-hexane was removed by low-temperature rotary evaporation under vacuum to obtain 2.69 g of a colorless oily substance with a strong mustard smell. The intermediate content was determined to be about 81% by GC-MS, and the yield of intermediate I was about 76.3%. Spectrum data is with embodiment 1.
...
Embodiment 3
[0061] (1) Dissolve 3.53g (20.6mmol) of 1-bromo-4-chlorobutane in 50ml of absolute ethanol, add to a 250ml round-bottomed flask, stir in an ice bath at 0°C, and slowly drop in the solution containing 3.47g (24.72mmol, 1.2 times the amount) of 50% sodium methyl mercaptide in absolute ethanol was naturally warmed to room temperature, overnight, and the total reaction time was 24h. Stirring was stopped, and the ethanol was removed by rotary evaporation at low temperature in vacuum, extracted three times continuously with n-hexane, washed with saturated sodium chloride successively, dried the extract with anhydrous sodium sulfate, and filtered off the insoluble matter. The n-hexane was removed by low-temperature rotary evaporation under vacuum to obtain 2.22 g of a colorless oily substance with a strong mustard smell. The intermediate content was determined to be about 57.6% by GC-MS, and the yield of intermediate I was about 44.8%. Spectrum data is with embodiment 1.
[0062] (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com