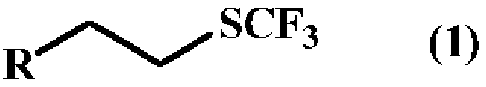Method for producing trifluoromethylthioalkyl compound
A technology of trifluoromethylthioalkyl and its manufacturing method, applied in the fields of thioether preparation, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Production of 6-(trifluoromethylthio)hexyl acetate
[0185] [chemical 5]
[0186]
[0187] Add 7.2 mL (94 mmol) of thiophosgene dropwise to 400 mL of acetonitrile containing 15.0 g (67.2 mmol) of 6-bromohexyl acetate and 23.4 g (403 mmol) of potassium fluoride over a period of 1 hour using a dropping funnel solution (the temperature in the reaction system at this time was 80° C.). After stirring the reaction mixture under reflux for 1 hour, the reaction mixture was naturally cooled to room temperature, and the insoluble matter was filtered off. Water was added to the filtrate, followed by extraction with diisopropyl ether, and the organic layer was washed with saturated aqueous sodium bicarbonate and saturated brine. The organic layer obtained by liquid separation was dried over anhydrous magnesium sulfate, and inorganic substances were filtered off, and the solvent was distilled off under reduced pressure. The obtained crude product was purified by vacuum distill...
Embodiment 2~4
[0191] Change the addition temperature and addition time respectively, dropwise add thiophosgene 0.60mL (7.7mmol) acetonitrile 4.5mL solution to 6-bromohexyl acetate 1.2g (5.5mmol), potassium fluoride 1.9g (33mmol) acetonitrile 32mL solution. After stirring for 1 hour, a part of the reaction mixture was analyzed by GC, and the yield of the target product was calculated by the area percentage method. The results are summarized in Table 1 in the same manner as in Example 1. In addition, in the GC analysis of Examples 2-4, the unreacted raw material compound (6-bromohexyl acetate) was detected other than the object.
[0192] [Table 1]
[0193]
Embodiment 5
[0195] Production of ethyl 6-(trifluoromethylthio)hexanoate
[0196] [chemical 6]
[0197]
[0198] Add 1.2 mL (15 mmol) of thiophosgene in 3.8 mL of acetonitrile dropwise to 62 mL of acetonitrile containing 2.4 g (11 mmol) of ethyl 6-bromohexanoate and 3.8 g (65 mmol) of potassium fluoride over 1 hour under heating to reflux solution (the temperature in the reaction system at this time was 80° C.). The reaction mixture was stirred under reflux for 1 hour, and then cooled to room temperature naturally. Water was added to the reaction mixture, followed by extraction with diisopropyl ether, and the organic layer was washed with saturated aqueous sodium bicarbonate and saturated brine. After the organic layer obtained by liquid separation was dried over anhydrous magnesium sulfate, the inorganic matter was filtered off, and the solvent was distilled off under reduced pressure to obtain 2.5 g of ethyl 6-(trifluoromethylthio)hexanoate as a reddish-brown liquid ( Yield 96%). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com