A method for preparation of perfluoroalkyl sulfenyl chloride
A technology of perfluoroalkylsulfinyl chloride and trifluoromethylthiomethylbenzene, applied in chemical methods for liquid-liquid reactions, preparation of sulfides, chemical instruments and methods, etc., capable of solving inherent dangers and insecurity , not commercially attractive, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
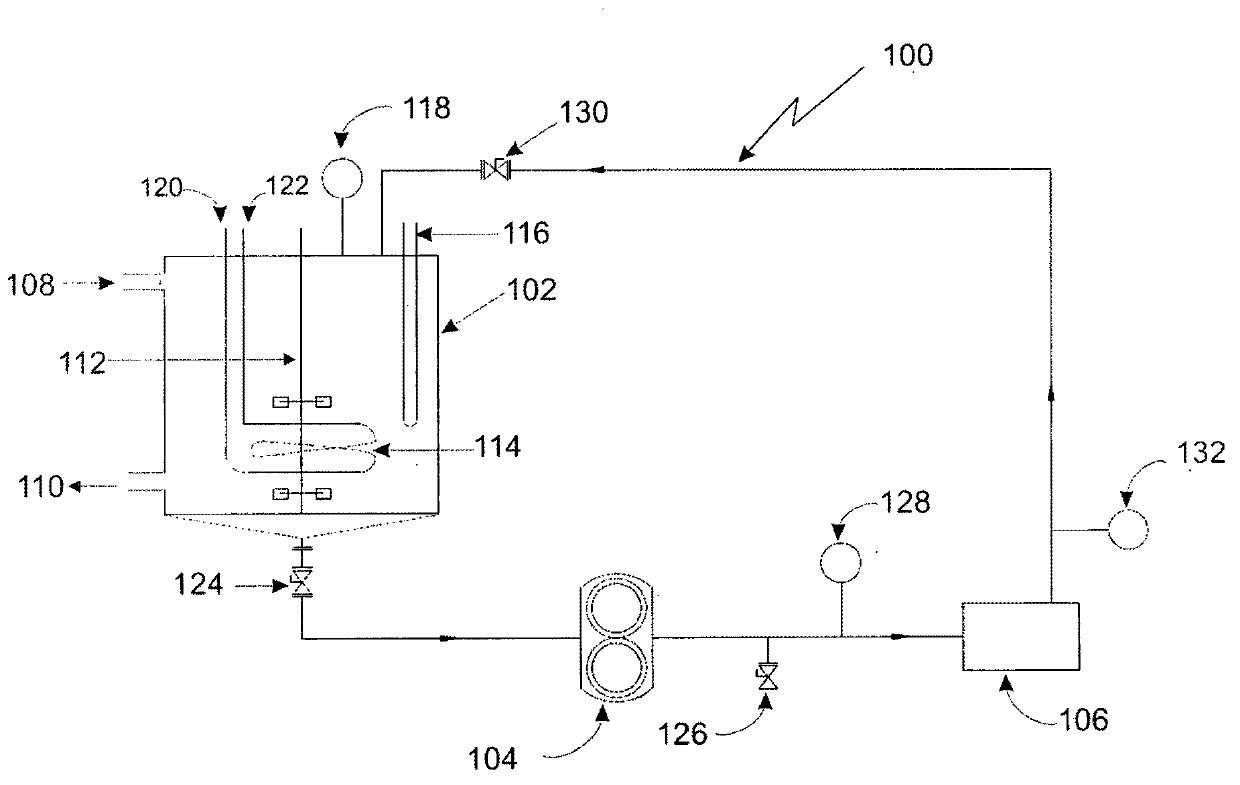
[0117]A glass reactor equipped with a central stirrer and a vertical condenser was charged with anhydrous acetonitrile (2.0 L), activated potassium fluoride (6.0 moles), potassium bifluoride (0.3 moles), and o-chlorobenzyl chloride ( 1.0 mol), and the obtained reaction mass was cooled to -15°C. After approximately 2 hours, low temperature thiophosgene (1.2 mol) was injected into the reaction mixture along with 300 mL of acetonitrile. The reaction mixture was then stirred for 2 hours, allowing the temperature of the mixture to rise to 0°C. The reaction mixture was stirred for a further 2 hours and the temperature was raised to 30°C, after which the mixture was kept at this temperature for 2 hours. 0.2 mol of thiophosgene was injected into the reaction mixture, the temperature of the mixture was raised to 80°C, kept at 80°C for 2 hours, and then cooled to 30°C. Additional thiophosgene (0.2 moles) was injected and the reaction temperature was raised to 80°C. Only 1% conversion...
example 2
[0119] A glass reactor equipped with a central stirrer and a vertical condenser was charged with anhydrous acetonitrile (2.0 L), tetramethylammonium fluoride (3.04 mol) and o-chlorobenzyl chloride (1.0 mol) to obtain The reaction mass was cooled to -40°C. After approximately 2 hours, low temperature thiophosgene (1.0 mol) was injected into the reaction mixture along with 300 mL of acetonitrile. The reaction mixture was stirred for 2 hours, the temperature of the mixture was raised to 0° C., and then the reaction mixture was stirred for an additional 2 hours. The temperature of the mixture was then raised to 30°C, and the mixture was kept at that temperature for 2 hours. Thiophosgene (0.2 mol) was injected into the reaction mixture and the temperature was raised to 80°C. The mixture was maintained at 80°C for 2 hours and then cooled to 30°C. Another 0.2 mole of thiophosgene was injected, and the temperature of the mixture was raised to 80°C. Only 2% conversion was observed ...
example 3
[0121] Charge anhydrous acetonitrile (2.0 L), activated potassium fluoride (6.0 mol), potassium bifluoride (0.3 mol), o-chlorobenzyl chloride (1.0 mol) into a glass reactor equipped with a central vertical stirrer and benzyltrimethylammonium fluoride (0.2 mol). The process steps in Example 1 were repeated. Only 1% conversion was observed after 10 hours. No increase in conversion was observed even after holding for several more hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com