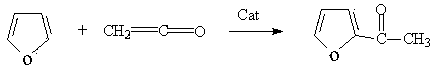Method for preparing 2-furyl-methylketon from ethenone
A technology of acetylfuran and ketene, applied in the production field of chemical intermediate 2-acetylfuran, which can solve the problems of low reactor production efficiency, low raw material utilization rate, and high production cost, and achieve low corrosion, low raw material cost, and three wastes The effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 136g furan, 1.0g phosphoric acid and 260g chloroform into a four-necked flask equipped with electric stirring, ketene gas tube, thermometer and vent tube, turn on the stirring, and pour 85g ketene within 3 hours at 15℃ . After recovering the solvent under normal pressure, rectifying under reduced pressure, collecting 78-81°C fractions at -0.099Mpa to obtain 210.2g of 2-acetylfuran with a purity of 99% (the yield is 95.5% based on furan).
[0024] After the solvent recovery is directly applied for 6 times, the effect of recovered solvent applied on the product yield and purity is shown in the following table:
[0025] Number of applications Yield(%) purity(%) 195.099.3 295.399.1 395.299.2 495.099.4 595.299.2 695.199.1
Embodiment 2
[0027] In a four-necked flask equipped with electric stirring, ketene gas tube, thermometer and exhaust tube, add 68g furan, 0.5g phosphoric acid, 150g dichloromethane, turn on the stirring, and pour ethylene within 3 hours at 20℃ Ketone 42.0g. After recovering the solvent at normal pressure, rectifying under reduced pressure, collecting 78-81°C fractions at -0.099Mpa to obtain 104.8 g of 2-acetylfuran with a purity of 99.1% (the yield is 95.2% based on furan). .
Embodiment 3
[0029] In a four-necked flask equipped with electric stirring, ketene gas tube, thermometer and exhaust tube, add 136g furan, 1.0g phosphoric acid, 280g methylene chloride, turn on the stirring, and pour ethylene within 3 hours at 20℃ Ketone 86g. After recovering the solvent under normal pressure, rectifying under reduced pressure, collecting 78-81°C fractions at -0.099Mpa to obtain 209 g of 2-acetylfuran with a purity of 99.3% (the yield is 95.0% based on furan).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com