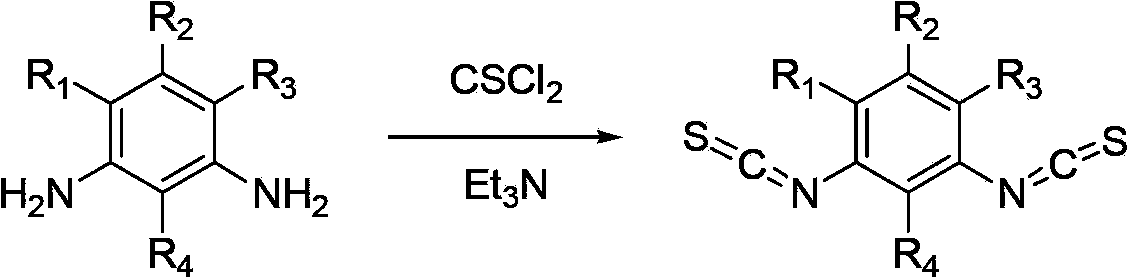
Method for synthesizing substituted m-phthalic isothiocyanate by one-pot method, and synthesized substituted m-phthalic isothiocyanate compound
A technology of m-phenylene diisothiocyanate and m-phenylenediamine, which is applied in the field of compound synthesis, can solve the problems of harsh reaction conditions, long reaction time and high production cost, and achieves mild reaction conditions, wide application and reduced cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0026] Reaction 1
[0027]
[0028] Compound 1: 2,4-Diisopentyloxym-phenylene diisothiocyanate
[0029] Structural formula:
[0030] Under anhydrous and oxygen-free conditions, the compound 2,4-diisopentyloxy m-phenylenediamine (269mg, 0.96mmol) and Et 3 A solution of N (1.3ml, 9.6mmol) in 11ml of dichloromethane was injected into (0.1ml, 1.3mmol) of thiophosgene in 60ml of dichloromethane, and the injection was completed in 1h. Afterwards, the reaction solution was placed at room temperature for 2 h. The reaction solution was washed successively with saturated sodium bicarbonate and saturated sodium chloride, dried over anhydrous sodium sulfate, and then subjected to petroleum ether / acetone column chromatography to obtain 311 mg of a colorless liquid, which was cooled to a white solid with a yield of 89.1%. 1 H NMR (400MHz, CDCl 3 )δ: 6.72(s,1H),6.37(s,1H),3.99(t,J=6.3Hz,4H),1.92-1.82(m,2H),1.70-1.65(m,4H),0.91(t ,J=6.6Hz,12H). 13 C NMR (100MHz, CDCl 3 )δ: 154.82,...
preparation Embodiment 2
[0042] Reaction 4
[0043]
[0044] Compound 4: n-butylamine 3,5-diisothiocyanate benzoate
[0045] Structural formula:
[0046]
[0047] Under anhydrous and oxygen-free conditions, the compound 3.5-diaminobenzoic acid n-butyramide (198mg, 0.96mmol) and Et 3 A solution of N (1.3ml, 9.6mmol) in 11ml of dichloromethane was injected into (0.1ml, 1.3mmol) of thiophosgene in 60ml of dichloromethane, and the injection was completed in 1h. Afterwards, the reaction solution was placed at room temperature for 2 h. The reaction solution was washed successively with saturated sodium bicarbonate and saturated sodium chloride, dried over anhydrous sodium sulfate, and then subjected to petroleum ether / acetone column chromatography to obtain 261 mg of a white solid with a yield of 93.4%. 1 H NMR (400MHz, CDCl 3 )δ: 7.47(d,J=1.8Hz,2H),7.13(t,J=1.8Hz,4H),6.15(s,1H),3.47-3.42(m,2H),1.64-1.57(m,2H ),1.45-1.36(m,2H),0.96(t,J=7.4Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ: 163.63, 138.26, 136...
preparation Embodiment 3
[0061] Reaction 7
[0062]
[0063] Compound 73, n-octyl 5-diisothiocyanate benzoate
[0064] Structural formula:
[0065]
[0066] Under anhydrous and oxygen-free conditions, at -75°C, the compound 3,5-diaminobenzoic acid n-octyl (253mg, 0.96mmol) and Et3N (1.3ml, 9.6mmol) in 11ml of dichloromethane were injected into (0.1ml, 1.3mmol) of thiophosgene in 60ml of dichloromethane, 1h injection was completed. Afterwards, the reaction solution was placed at room temperature for 2 h. The reaction solution was washed successively with saturated sodium bicarbonate and saturated sodium chloride, dried over anhydrous sodium sulfate, and then subjected to petroleum ether / acetone column chromatography to obtain 321 mg of a colorless liquid, which was cooled to a white solid with a yield of 96.1%. 1 H NMR (400MHz, CDCl 3 )δ7.75(d,J=1.9Hz,2H),7.20(t,J=1.9Hz,1H),4.33(t,J=6.7Hz,2H),1.84–1.70(m,2H),1.41- 1.29(m,10H),0.89(t,J=6.8Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ: 164.04, 139.32,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com