Method for preparing sulfonyl ring thiourea from mono-sulfonyl diamine in aqueous phase
A technology of monosulfonyl diamine water and sulfonyl cyclic thiourea, which is applied in the field of preparation of sulfonyl cyclic thiourea compounds, can solve the problem of low yield, high price of thiocarbonyldiimidazole, and thiophosgene To solve the problem of high toxicity, achieve the effect of simple method, easy access to raw materials, and simplified preparation process
Inactive Publication Date: 2010-11-10
TIANJIN UNIV
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
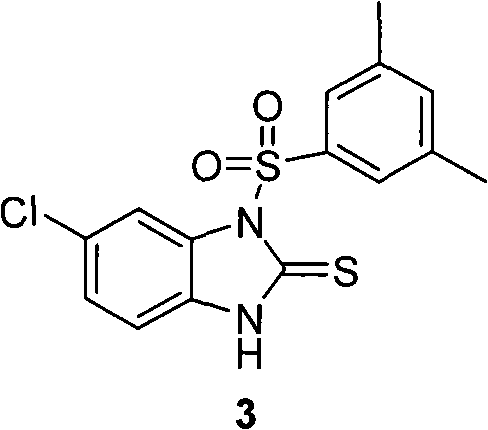
The used thiophosgene toxicity of this method is high (referring to BioorganicandMedicinalChemistry, 2010,18 (4), 1702-1710), and the used thiocarbonyldiimidazole price is higher (referring to US2005038076), and reaction needs to carry out in organic solvent, And the yield is not high
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
The invention discloses a method for preparing sulfonyl ring thiourea from mono-sulfonyl diamine in an aqueous phase, and belongs to a preparative technique of sulfonyl ring thiourea compounds. The method comprises the following processes of: adding mono-sulfonyl diamine, carbon disulfide, and one of sodium hydroxide, potassium hydroxide, sodium carbonate and potassium carbonate into water according to the molar ratio of the mono-sulfonyl diamine to the carbon disulfide to alkali or the molar ratio of the mono-sulfonyl diamine to the carbon disulfide to carbonate, performing reaction with stirring to obtain a compound, and filtering and washing the compound to obtain the sulfonyl ring thiourea. The method has the advantages of overcoming numerous defects in the prior art and avoiding thiophosgene or thiocarbonyl diimidazole; and the reaction takes the water as a medium, so the method also has the advantages of no need of organic solvent and heating, simpleness, convenience, readily available raw materials, and environmental friendliness.
Description
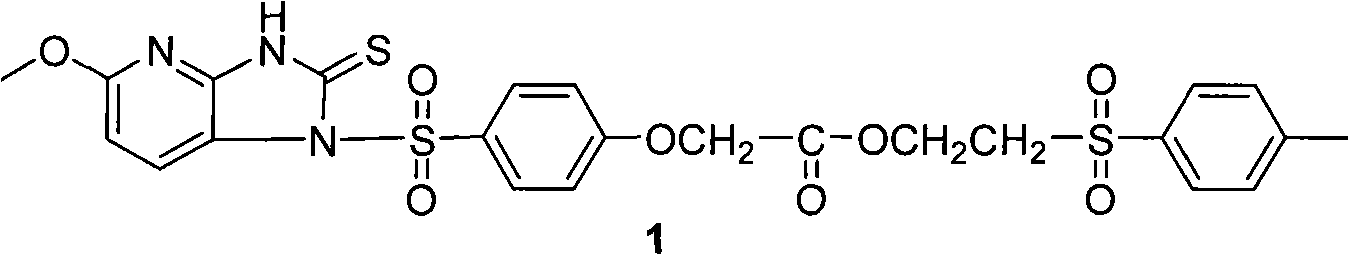
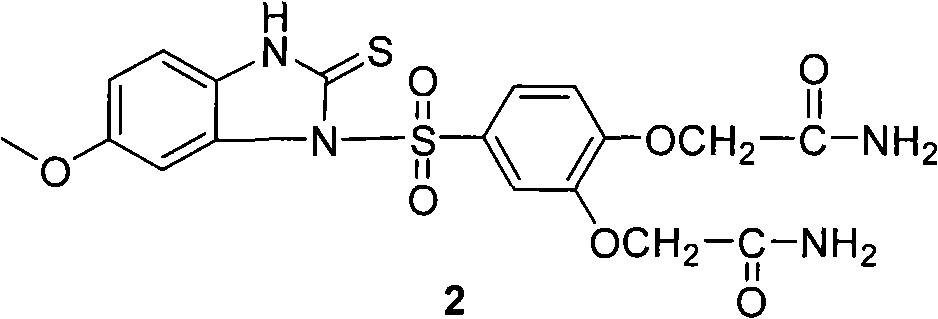
Method for preparing sulfonyl cyclic thiourea from monosulfonyl diamine aqueous phase technical field The invention relates to a method for preparing sulfonyl cyclic thiourea from monosulfonyl diamine aqueous phase, which belongs to the preparation technology of sulfonyl cyclic thiourea compounds. Background technique Sulfonyl cyclic thiourea compounds have a variety of uses. For example, US Patent US2005038076 reports that compound 1 can be used as a precursor of anti-ulcer drugs: World patent WO2008036201 reports that compound 2 has a better effect. US Patent US20090163545 reports that certain compounds containing sulfonyl cyclic thiourea structures can increase the lifespan of eukaryotic organisms, and on this basis, drugs for health care or life extension can be developed. It is reported that compound 3 and its analogues having the following structure have inhibitory effect on HIV-1 virus (see Bioorganic and Medicinal Chemistry, 2010, 18(4), 1702-1710). ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07B45/00C07D233/42C07D239/10C07D235/28
Inventor 马宁马晓思万国翔
Owner TIANJIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com