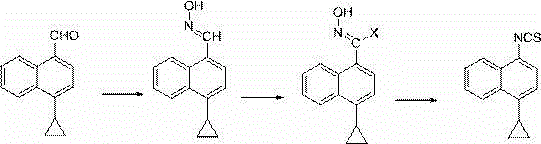
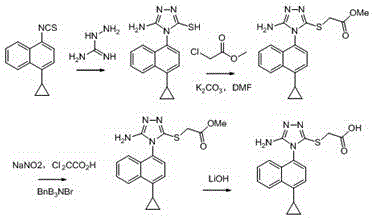
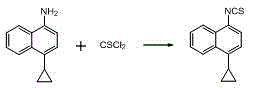A method for synthesizing 4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione
A technology of isothiocyanatonaphthalene and cyclopropyl, which is applied in the field of synthesis of 1-cyclopropyl-4-isothiocyanatonaphthalene, can solve the problem of high cost, complex process, prolongation of 1-cyclopropyl-4- Problems such as the synthesis route of isothiocyanatonaphthalene, to achieve the effects of reducing the amount of production, simple reaction process, stable reaction and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 100 mL of toluene, 18.3 g (0.100 mol) of 4-cyclopropyl-1-naphthylamine, and 33.7 g (0.3 mol) of triethylenediamine into a 250 mL three-necked flask, and stir at room temperature. Add 22.8 g (0.300 mol) of carbon disulfide dropwise, and stir at room temperature for 4-10 hours after the addition, and a large amount of solids precipitate out. After suction filtration and drying, 33.5 g of 4-cyclopropyl-1-naphthylaminodithioformic acid triethylenediamine salt was obtained, with a yield of 90.1%;
[0028] Add 33.5g (0.090 mol) of the solid product from the previous step reaction into three 250mL flasks, add 100mL of chloroform, stir and cool down to 5~10°C, slowly add 9.9 g (0.033mol) of BTC in chloroform solution dropwise, after the addition is complete The reaction was stirred at room temperature for 1 hour, and after the reaction was completed, the temperature was raised to 40-65° C. for reflux reaction for 1 hour. After cooling to room temperature, the insoluble mat...
Embodiment 2
[0030] Add 100 mL of xylene, 18.3 g (0.100 mol) of 4-cyclopropyl-1-naphthylamine, and 30.4 g (0.300 mol) of triethylamine into a 250 mL three-necked flask, and stir at room temperature. 22.8 g (0.300 mol) of carbon disulfide was added dropwise, and stirred at room temperature for 4-10 hours after the addition was complete, a large amount of solids precipitated. After suction filtration and drying, 31.8 g of 4-cyclopropyl-1-naphthylaminodithioformic acid triethylamine salt was obtained, with a yield of 88.2%;
[0031] Add 31.8 g (0.088 mol) of the solid product from the previous step reaction into three 250 mL flasks, add 100 mL of dichloromethane, stir and cool down to 5~10°C, slowly add 9.9 g (0.033 mol) of BTC in chloroform solution dropwise, add After the completion, the reaction was stirred at room temperature for 1 hour, and after the reaction was completed, the temperature was raised to 40-65° C. for reflux reaction for 3 hours. After cooling to room temperature, the in...
Embodiment 3
[0033] Add 100 mL of xylene, 18.3 g (0.100 mol) of 4-cyclopropyl-1-naphthylamine, and 23.7 g (0.300 mol) of pyridine into a 250 mL three-necked flask, and stir at room temperature. 22.8 g (0.300 mol) of carbon disulfide was added dropwise, and stirred at room temperature for 4-10 hours after the addition was complete, a large amount of solids precipitated. After suction filtration and drying, 29.5 g of 4-cyclopropyl-1-naphthylaminopyridinium dithiocarbamate was obtained, with a yield of 87.1%;
[0034] Add 29.5 g (0.087 mol) of the solid product of the previous step reaction into three 250 mL flasks, add 100 mL of chloroform, stir and cool down to 5~10°C, slowly add dichloromethane dissolved in 9.8 g (0.090 mol) of ethyl chloroformate After the addition of the solution, stir and react at room temperature for 1 hour, and then heat up to 40~65°C for reflux for 3 hours after the reaction. After cooling to room temperature, the insoluble matter was removed by suction filtration, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com