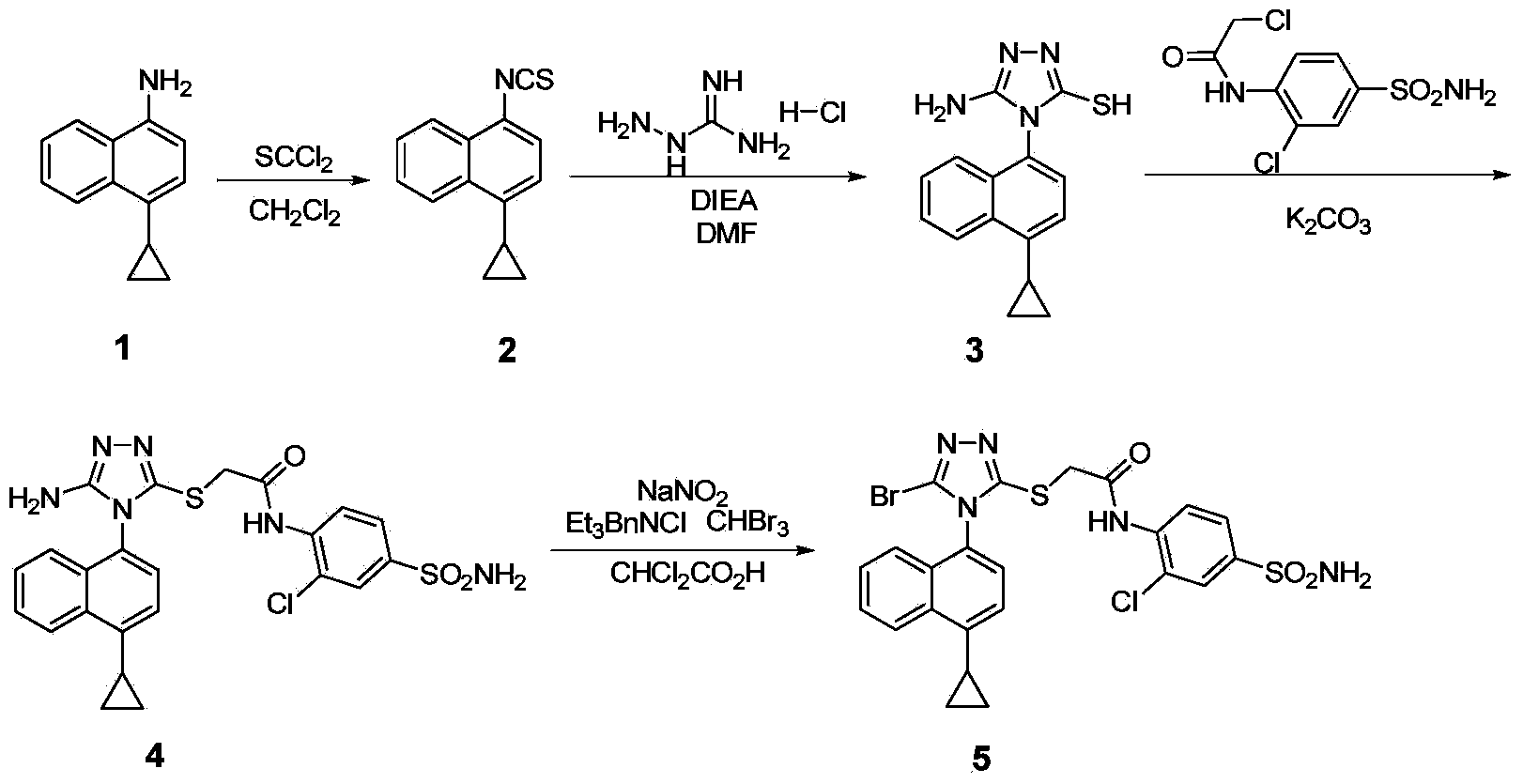
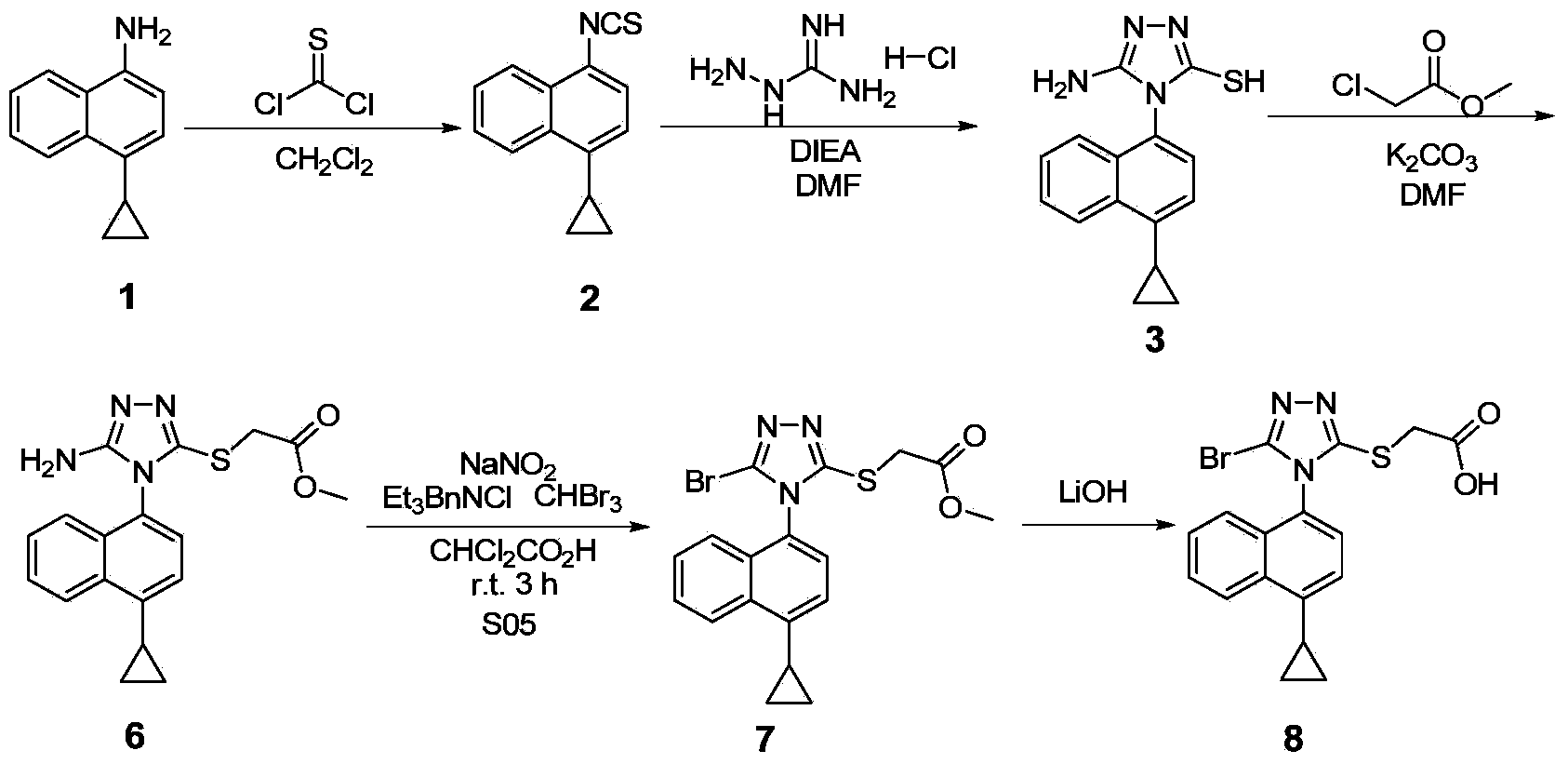
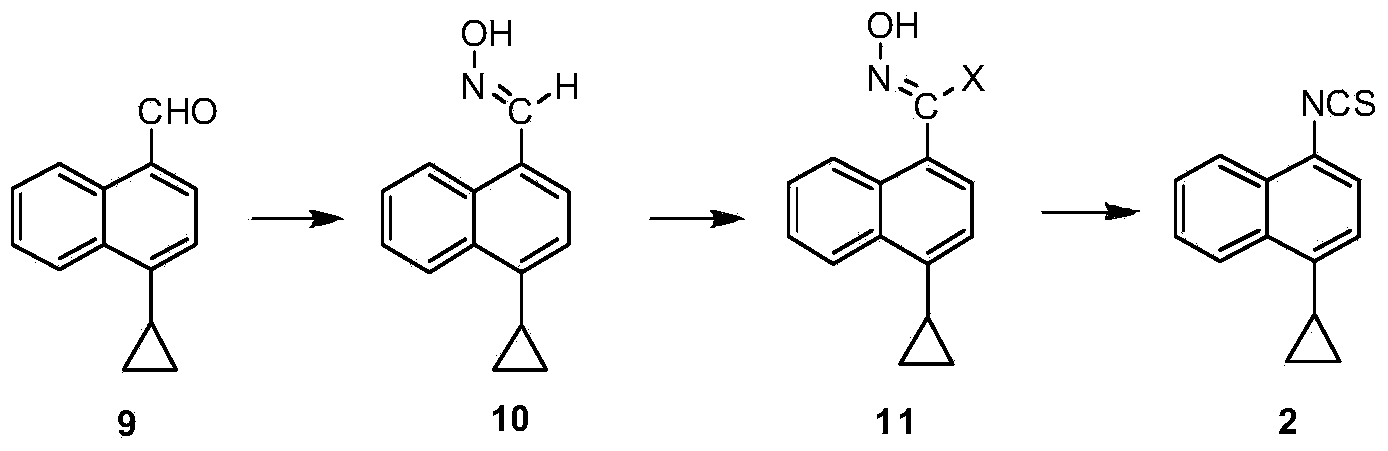
Preparation method of gout curative medicine Lesinurad and midbody of Lesinurad
An intermediate and representative technology, applied in the field of preparation of gout treatment drug Lesinurad, can solve the problems of complicated operation, expensive raw materials and high cost, and achieve the effects of simple operation, easy separation and purification, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 prepares compound 12 by compound 1
[0063]
[0064] In a single-necked bottle, add 4-cyclopropyl-1-naphthylamine (compound 1, 1.0eq), add DMF (8.2Vol) to dissolve it, add NaOH (1.2eq) and CS 2 (1.2eq), stirred and reacted at room temperature for 1h, added hydrazine hydrate (3.0eq) and transferred to 70°C oil bath, stirred and refluxed for 4h, LC confirmed that the reaction was complete, stopped heating and stirring, added 10mL water per 1g of raw material after cooling, and suction filtered , the filter cake was recrystallized with 95% ethanol, suction filtered, and the solid was dried to obtain compound 12 with a yield of 50%-70%.
[0065] The NMR data of the product are as follows: 1 H NMR (400MHz,DMSO)δ9.16(s,1H),8.43(d,J=7.8Hz,1H),7.98–7.89(m,1H),7.66-7.48(m,3H),7.26(d, J=7.6Hz,1H),5.54–4.50(m,1H),2.46–2.34(m,1H),1.12–1.02(m,2H),0.80–0.69(m,2H).
Embodiment 2
[0066] Embodiment 2 prepares compound 13 by compound 12
[0067]
[0068] Add compound 12 (1.0eq) in the single-necked bottle, add 1,4-dioxane (7.5Vol) to make it dissolve, add N,N-dimethylformamide dimethyl acetal (1.0eq), at 100 Stir and reflux at ℃ for 1.5-2h, LC confirmed the completion of the reaction, after cooling the reaction solution to room temperature, add 10mL of water to 1g of the raw material, the precipitated solid was suction filtered, the filter cake was washed twice with 95% ethanol, and the solid was dried to obtain compound 13【Lesinurad Intermediate, chemical name: 4-(4-cyclopropylnaphthalene)-3-mercapto-1,2,4-triazole)]. The yield is 70%-80%.
[0069] The NMR data of the product are as follows: 1 H NMR(400MHz,DMSO)δ8.69(s,1H),8.57(d,J=8.4Hz,1H),7.72(t,J=7.3Hz,1H),7.64(t,J=7.4Hz,1H ),7.54(d,J=7.6Hz,1H),7.44–7.37(m,2H),2.56–2.50(m,1H),1.19-1.09(m,2H),0.90–0.75(m,2H).
Embodiment 3
[0070] Embodiment 3 prepares compound 15 by compound 13
[0071]
[0072] The NMR data of the product are as follows: Compound 13 (4g, 1eq) was added to a single-necked bottle, and the solvent DMF (67mL, 16.8vol) was added to dissolve, stirred at room temperature, and K 2 CO 3 (2.272g, 1.1eq), then add compound 14 (1.74mL, 1.05eq) dropwise, control the drop rate at 0.5mL / min, stir at room temperature for 2h, the reaction is complete, add EA (ethyl acetate) and water to dilute the reaction solution, extracted three times with EA, washed three times with saturated brine, anhydrous MgSO 4 dry. The organic phase was spin-dried to obtain a white solid [that is, compound 15, a Lesinurad intermediate, chemical name: 4-(4-cyclopropylnaphthalene)-3-ethyl thioacetate-1,2,4-triazole], After drying, 5.13 g was obtained, and the yield was 97%.
[0073] The NMR data of the product are as follows: 1 H NMR (400MHz, CDCl 3 )δ8.54(d,J=8.4Hz,1H),8.30(s,1H),7.70-7.62(m,1H),7.61-7.53(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com