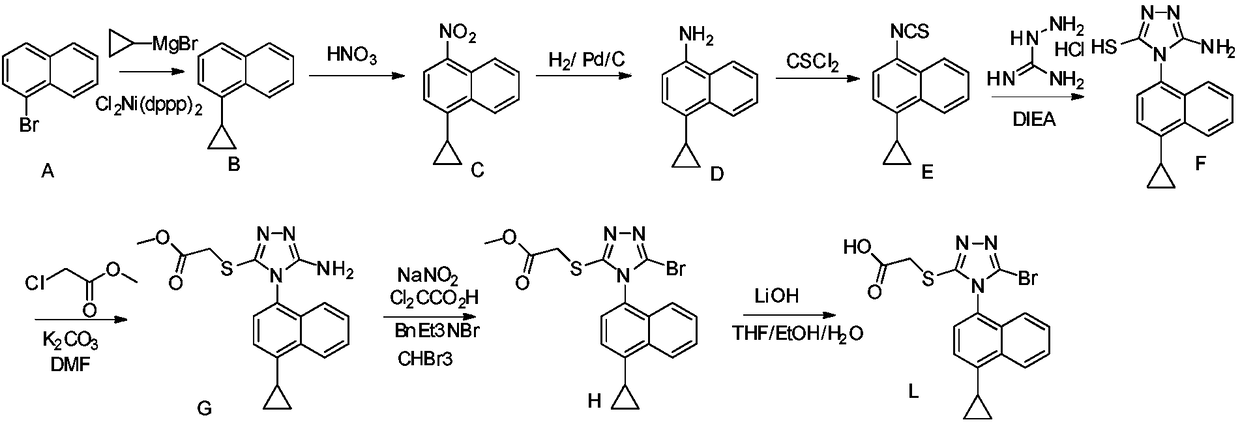
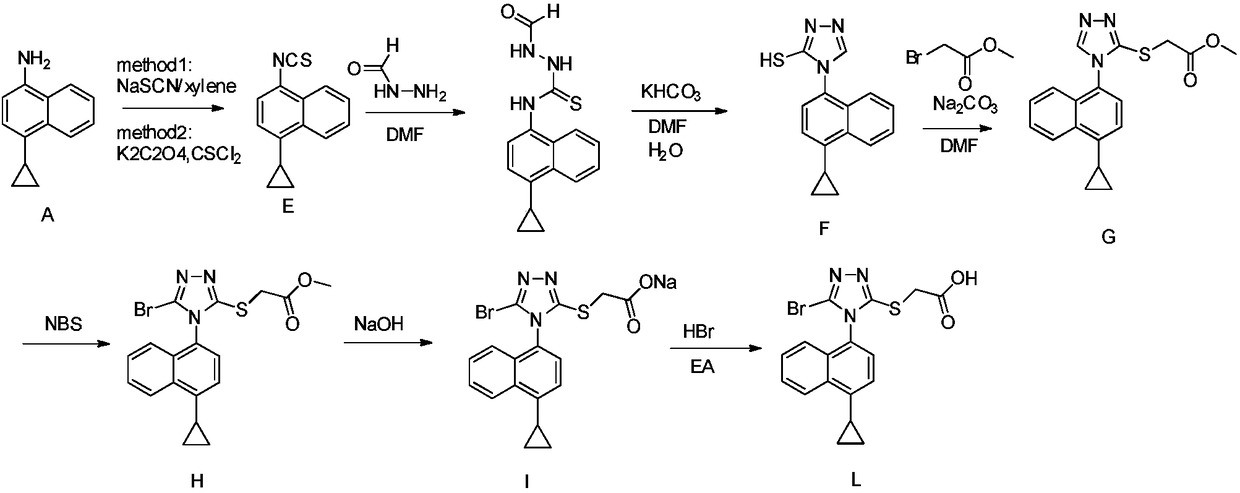
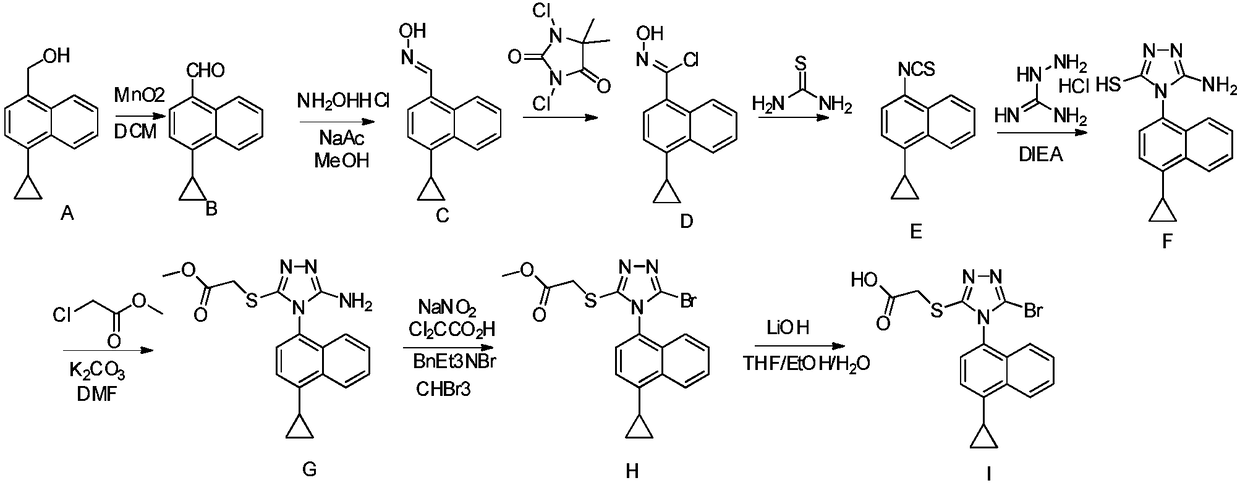
New preparation method of anti-gout medicine Lesinurad and its key intermediate
A technology of intermediate and general formula, applied in the field of pharmaceutical synthesis, can solve the problems of high production cost, complicated operation, expensive raw materials or reagents, etc., and achieve the effects of simple operation and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of 4-(4-cyclopropylnaphthalene)-1,2,4-triazole
[0049]
[0050] In the three-necked flask, add 4-cyclopropyl-1-naphthylamine (compound 1, 110.00mmol), diformyl hydrazide (330.00mmol) and pyridine (10V), and slowly add trimethylchlorosilane dropwise at room temperature (550mmol), the reaction was then heated to reflux for 2 hours. After LC confirms that the reaction is complete, remove the insoluble solid salt by filtration, concentrate the filtrate to dryness, add ethyl acetate to dissolve the obtained residue, wash the organic phase twice with water, dry the organic phase, concentrate under reduced pressure to about 30ml, and add methyl alcohol to the concentrated solution. 90ml of tert-butyl ether, the resulting suspension was beaten and stirred for 1 hour, and filtered with suction to obtain compound 2 (purity: 98%), yield (70%).
[0051] 1 H NMR (400MHz, CDCl 3)δ8.56(d, J=8.4Hz, 1H), 8.41(s, 2H), 7.70-7.66(m, 1H), 7.60-7.56(m, 1H), 7.44...
Embodiment 2
[0052] Example 2: Preparation of 4-(4-cyclopropylnaphthalene)-3,5-dibromo-1,2,4-triazole
[0053]
[0054] In the three-necked flask, add 4-(4-cyclopropylnaphthalene)-1,2,4-triazole (compound 2, 48.91 mmol), tetrahydrofuran (6V), and add N-bromo Succinimide (122.28 mmol). The reaction was then stirred at 40°C for 2 hours. After LC confirms that the reaction is over. The reaction solution was diluted with ethyl acetate, and the organic phase was washed twice with 30% sodium thiosulfate and saturated sodium bicarbonate solution, dried and concentrated. Add 40ml of methyl tert-butyl ether to the residue, stir and beat the suspension for 1 hour, filter with suction, wash the filter cake twice with 10ml of methyl tert-butyl ether to obtain compound 3 (purity: 99%), yield (85% )
[0055] 1 H NMR (400MHz, CDCl 3 )δ8.58(d,J=8.4Hz,1H),7.71-7.67(m,1H),7.62-7.58(m,1H),7.41(d,7.6Hz,1H),7.35(d,7.6Hz, 1H), 7.18(d, J=8.4Hz, 1H), 2.47-2.44(m, 1H), 1.21-1.18(m, 2H), 0.92-0.88(m, 2H);...
Embodiment 3A
[0056] Example 3A: Preparation of 4-(4-cyclopropylnaphthalene)-3-methyl thioacetate-5-bromo-1,2,4-triazole
[0057]
[0058] In the three-necked flask, add 4-(4-cyclopropylnaphthalene)-3,5-dibromo-1,2,4-triazole (compound 3, 10.18mmol), N,N-dimethylformamide (10V), at room temperature, potassium carbonate (15.26mmol) and methyl thioglycolate (15.26mmol) were added successively. The reaction was stirred at room temperature for 1 hour, and the reaction of the raw materials was detected by LC. Add ethyl acetate to dilute the reaction solution, wash the organic phase once with 0.5N hydrochloric acid solution, then wash three times with water, dry and concentrate to obtain the crude product of 4, and separate through a silica gel column to obtain compound 4 (purity: 90%), the yield ( 50%)
[0059] 1 H NMR (400MHz, CDCl 3 )δ8.55(d, J=8.4Hz, 1H), 7.68-7.64(m, 1H), 7.60-7.56(m, 1H), 7.36(s, 2H), 7.26(d, J=8.4Hz, 1H ),4.09(d,J=16.4Hz,1H),4.03(d,J=16.4Hz,1H),3.72(s,3H),2.45-2.41...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com