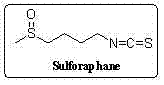
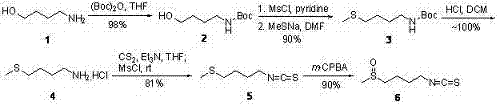Synthetic method for sulforaphane
A technology of sulforaphane and methylthiobutyl, which is applied in the field of synthesis of natural product sulforaphane, can solve the problems of cumbersomeness, high cost, high toxicity, etc., and achieve the effect of avoiding hydrazinolysis reaction and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
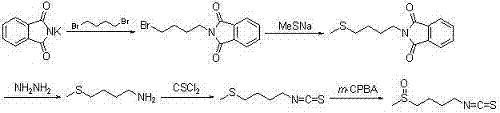
Examples
Embodiment 1
[0023] Step 1: 4-tert-butoxyamide-1-butanol 2 preparation of
[0024] Embodiment 1: 4.8 grams (54 millimoles) 4-amino-1-butanols are dissolved in 80 milliliters of tetrahydrofuran, add 12.4 grams (56.7 millimoles) di-tert-butoxycarbonic anhydride (Boc) at room temperature 2 O, stirred for 30 minutes. TLC analysis showed complete disappearance of starting material. After removing the solvent with a rotary evaporator, the residue was separated by flash silica gel column chromatography (eluent: 10% ethyl acetate / n-hexane) to obtain 10.1 g (yield 98%) of 4-tert-butoxyamide-1- Butanol 2。 The product was a colorless transparent liquid, which solidified after standing at room temperature and became a white solid. 1 H NMR (300 MHz, CDCl 3 ): d1.37 (s, 9H), 1.50 (m, 4H), 2.95 (br s, 1H), 3.04 (m, 2H), 3.56 (m, 2H), 4.82 (br s, 1H); 13 C NMR (75 MHz, CDCl 3 ): d26.71, 28.59, 29.87, 40.48, 62.23, 79.30, 156.47; MS (ESI): m / z 190 (M+1), 212 (M+Na).
[0025] 15.2 g (80 mmol) of ...
Embodiment 2
[0033] Step 1: 4-tert-butoxyamide-1-butanol 2 preparation of
[0034] Embodiment 2: 2.4 grams (27 millimoles) of 4-amino-1-butanol are dissolved in 40 milliliters of tetrahydrofuran, and 4.38 grams (20 millimoles) of di-tert-butoxycarbonic anhydride (Boc) are added at room temperature 2 O, stirred for 30 minutes. After removing the solvent with a rotary evaporator, the residue was separated by flash silica gel column chromatography (eluent: 10% ethyl acetate / n-hexane) to obtain 8.36 g (yield 82%) of 4-tert-butoxyamide-1- Butanol 2。 product of 1 H NMR, 13 C NMR and mass spectrum are exactly the same as Example 1.
[0035] Step 2: 4-tert-butoxyamidobutyl-1-methylsulfide 3 preparation of
[0036] Example 2: 15.2 grams (80 mmol) of 4-tert-butoxyamide-1-butanol 2 , a mixture of 16.7 ml (120 mmol) of triethylamine and 240 ml of anhydrous dichloromethane was cooled to 0°C with an ice-water bath, and 6.22 ml (80 mmol) of methanesulfonyl chloride was added dropwise with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com