Method for preparing N-methyl-N-(3-methyl phenyl) thiocarbamate-2-naphthyl
A technology of thiocarbamic acid and methyl phenyl, which is applied in organic chemistry and other fields, and can solve problems such as restrictions on industrial production and safety issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
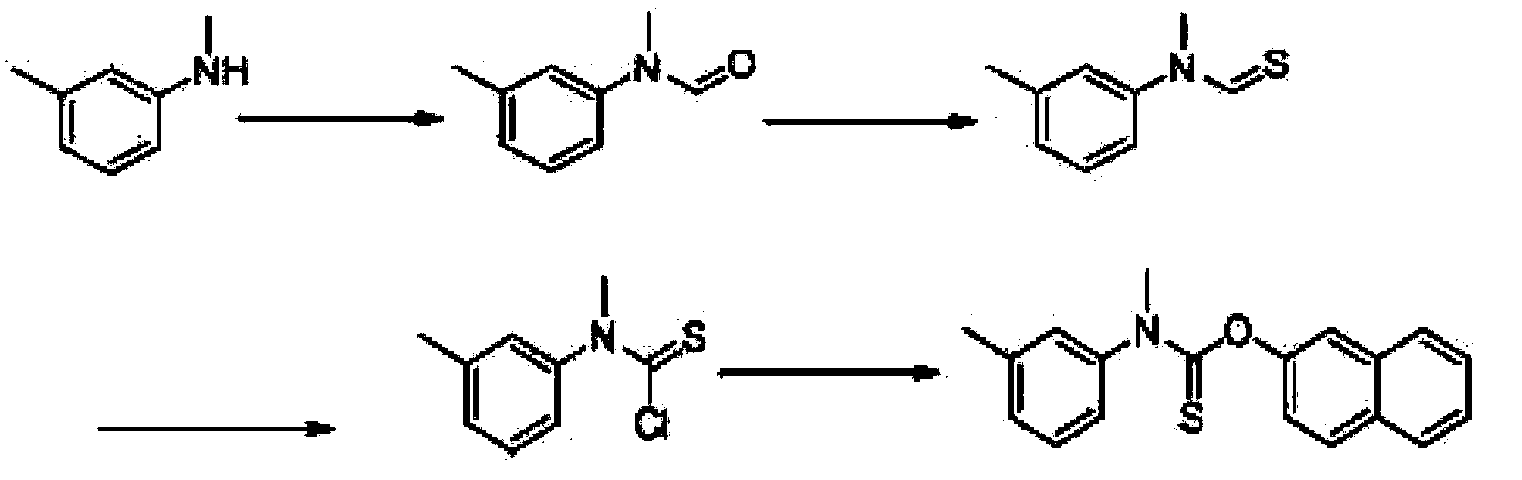
[0032] A preparation method of compound N-methyl-N-(3-methylphenyl)thiocarbamate-2-naphthyl ester as shown in formula E, is characterized in that: the method comprises the following steps:
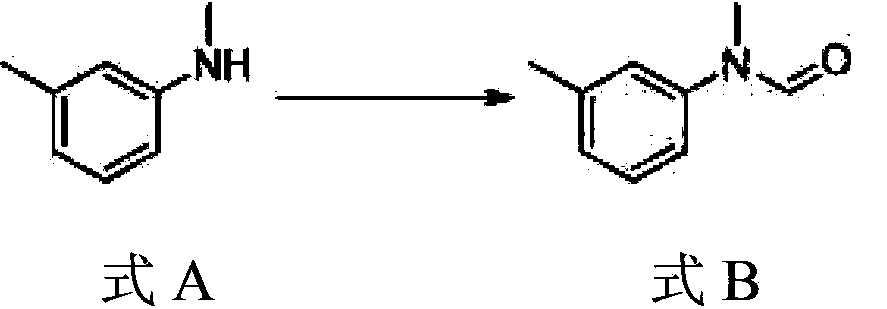
[0033] 1) Preparation of compound N-methyl-N-(3-methylphenyl) formamide shown in formula B
[0034] Take 20g of the compound N-methyl-3-methylaniline shown in formula A and 1.0g of active zinc powder, add it into a 100ml three-necked flask, add 22ml of formic acid dropwise under stirring, and control the temperature at 40°C. After the dropwise addition, Raise the temperature to reflux reaction, then lower to room temperature after completion, add 50ml of dichloromethane, filter, wash with a small amount of dichloromethane, combine the organic phase, wash the organic phase twice with water, twice with saturated sodium bicarbonate solution, and twice with saturated brine Once concentrated under reduced pressure, 23g of compound N-methyl-N-(3-methylphenyl)formamide as shown in formula B was o...
Embodiment 2
[0046] A preparation method of compound N-methyl-N-(3-methylphenyl)thiocarbamate-2-naphthyl ester as shown in formula E, is characterized in that: the method comprises the following steps:
[0047] 1) Preparation of compound N-methyl-N-(3-methylphenyl) formamide shown in formula B
[0048] Take 19g of the compound N-methyl-3-methylaniline shown in formula A, add 30g of toluene into a 50ml three-necked flask, stir, add 8.8g of formic acid dropwise at room temperature, after the dropwise addition, heat up to reflux for reaction, and the reaction is complete Then it was cooled to room temperature, washed with water to neutrality, and the solvent was recovered to obtain 21 g of compound N-methyl-N-(3-methylphenyl)formamide shown in formula B;
[0049]
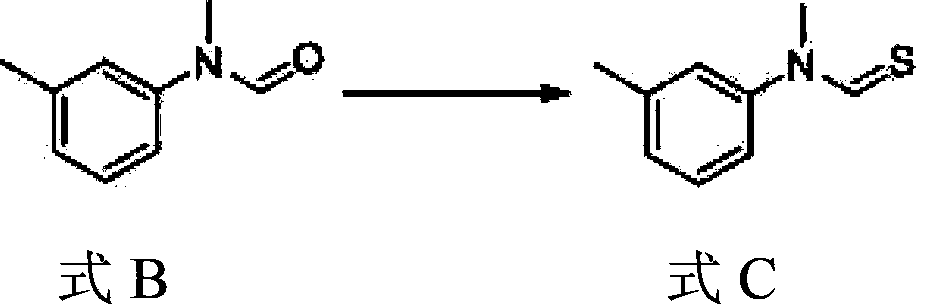
[0050] 2) Preparation of compound N-methyl-N-(3-methylphenyl) thioformamide shown in formula C
[0051] Take 21g of the compound N-methyl-N-(3-methylphenyl)formamide shown in formula B, and add 50ml of toluene into a 200ml three-...
Embodiment 3
[0060] A preparation method of compound N-methyl-N-(3-methylphenyl)thiocarbamate-2-naphthyl ester as shown in formula E, is characterized in that: the method comprises the following steps:
[0061] 1) Preparation of compound N-methyl-N-(3-methylphenyl) formamide shown in formula B
[0062] Take 19g of the compound N-methyl-3-methylaniline shown in formula A, add 30g of toluene into a 50ml three-necked flask, stir, add 8.8g of formic acid dropwise at room temperature, after the dropwise addition, heat up to reflux for reaction, and the reaction is complete Then it was cooled to room temperature, washed with water to neutrality, and the solvent was recovered to obtain 21 g of compound N-methyl-N-(3-methylphenyl)formamide shown in formula B;
[0063]
[0064] 2) Preparation of compound N-methyl-N-(3-methylphenyl) thioformamide shown in formula C
[0065] Take 21g of the compound N-methyl-N-(3-methylphenyl)formamide shown in formula B, and add 50ml of toluene into a 200ml thre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com