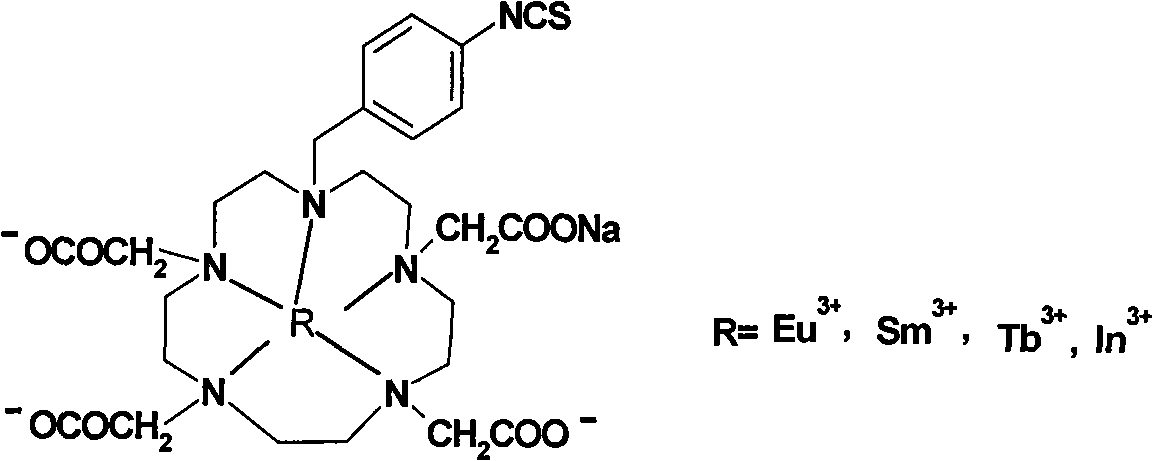
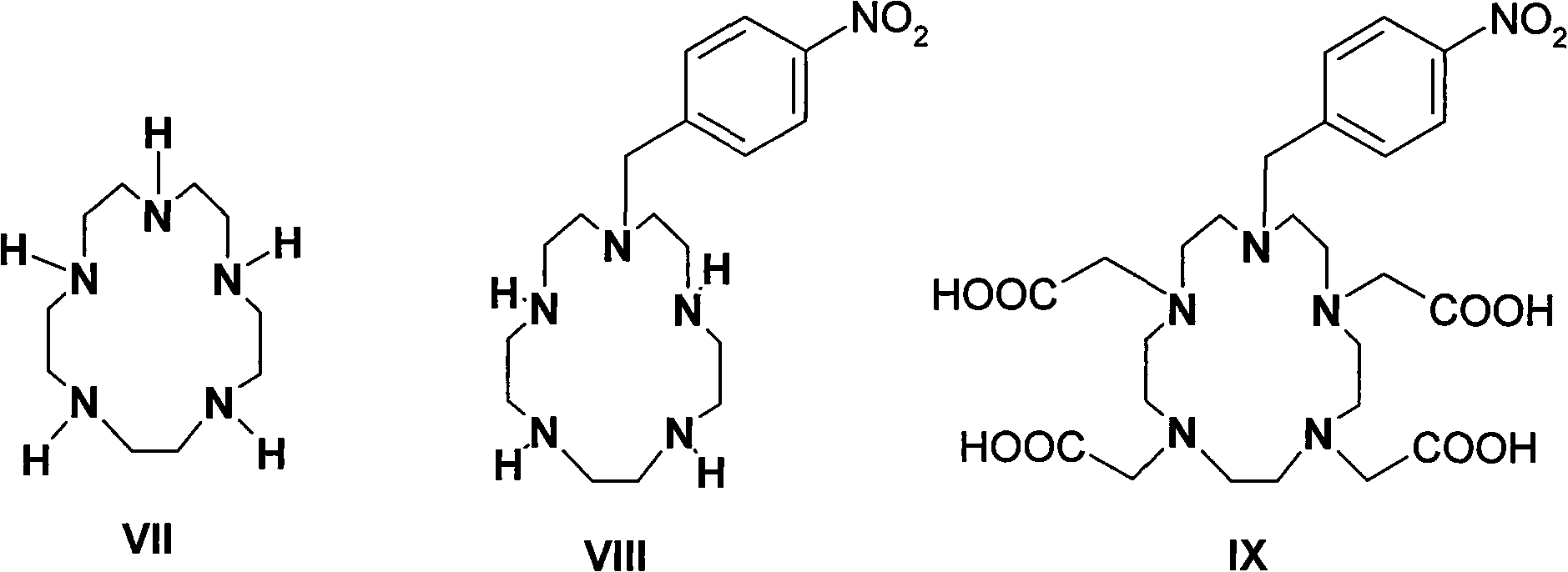
Para-thiocyano benzyl five-nitrogen heterocyclic ring tetraacethyl chelate and preparation method thereof
A kind of technology of isothiocyanatobenzyl pentaazine, chelate compound, applied in the application of immunoassay, p-isothiocyanatobenzyl pentaazacyclotetraacetic acid chelate compound and preparation thereof, homogeneous time-resolved fluorescence Immunoassay reagent field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
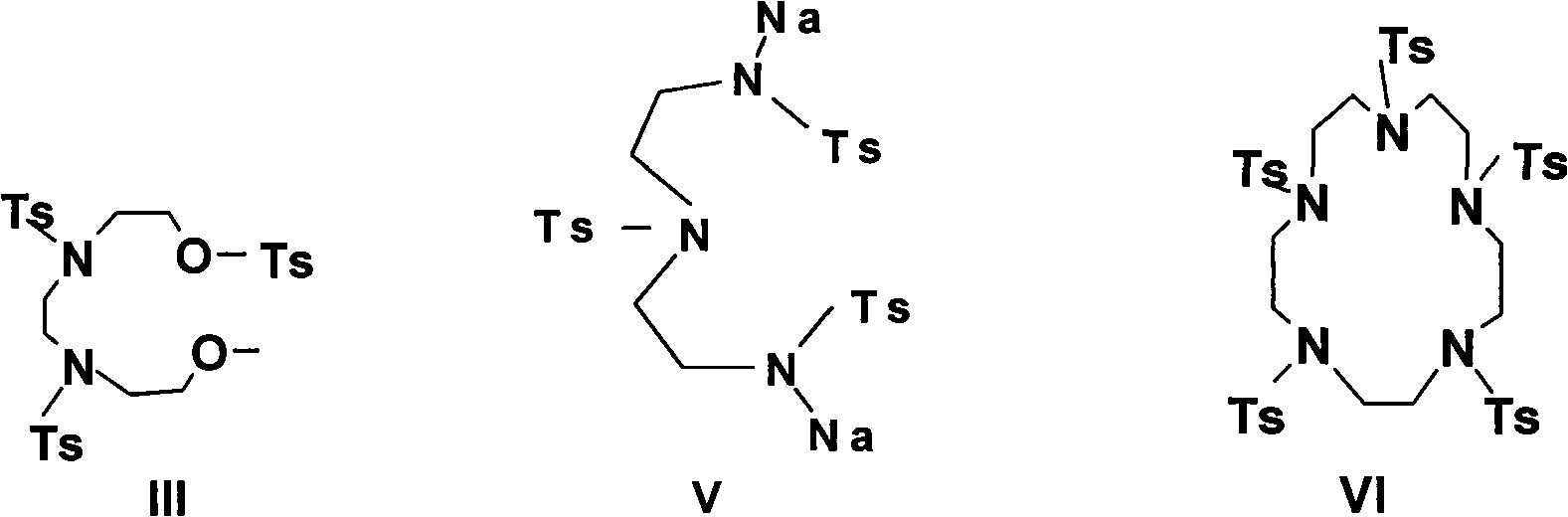
[0038] N, N'-di-p-toluenesulfonyl hydroxyethyl-N, N'-di-p-toluenesulfonyl ethylenediamine (compound III)
[0039] 77g (0.4mol) of p-toluenesulfonyl chloride in 100ml of pyridine, under vigorous stirring, was added dropwise a solution of 14.8g (0.1mol) of N, N'-di(2-hydroxyethyl)ethylenediamine in 200ml of pyridine, dropwise Stir at room temperature for 3 hours. Pour the solution into 250ml of ice and 250ml of concentrated hydrochloric acid to filter, wash with water, and recrystallize from ethanol to obtain pale yellow crystals, mp: 99-100°C (70%).
Embodiment 2
[0041] N, N', N"-tri-p-toluenesulfonyldiethylenetriamine (Compound IV)
[0042] Add 10.3g (0.1mol) of diethylenetriamine to 106ml (0.4mol) of sodium hydroxide solution, and under vigorous stirring, add dropwise 57.1g (0.3mol) of p-toluenesulfonyl chloride in 375ml of diethyl ether solution at 0°C, and the addition is complete. Continue to stir for 1 hour, filter to obtain the crude product, recrystallize with ethanol, mp: 200-202 ° C (60%)
Embodiment 3
[0044] N, N', N"-tri-p-toluenesulfonyldiethylenetriamine disodium salt (Compound V)
[0045] 56.4g (0.1mol) of compound 2 was added to 13.6g (0.2mol) of sodium ethoxide and heated in 150ml of absolute ethanol solution at 80°C for 1 hour, the solvent was removed under reduced pressure, and the product was obtained by vacuum drying. Preparation Example
[0046] Example 1
[0047] 1,4,7,10,13-penta-toluenesulfonylpentaazacyclopentadecane (compound VI)
[0048] First add 100ml of DMF, then blow nitrogen, heat at 100°C, and slowly add dropwise a solution of 76.4g (0.1mol) of compound III in 200ml of DMF and a solution of 61g (0.1mol) of compound V in 200ml of DMF while stirring. After dropping, brown The reaction solution was reacted at 100-110° C. for 3 hours. Cool, stir, pour into water, filter out the precipitate, wash with water, wash with alcohol, and recrystallize with ethanol to obtain white crystals mp: 289-290°C (document: 290-291°C, 278-280°C) (65%).
[0049] Example ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com