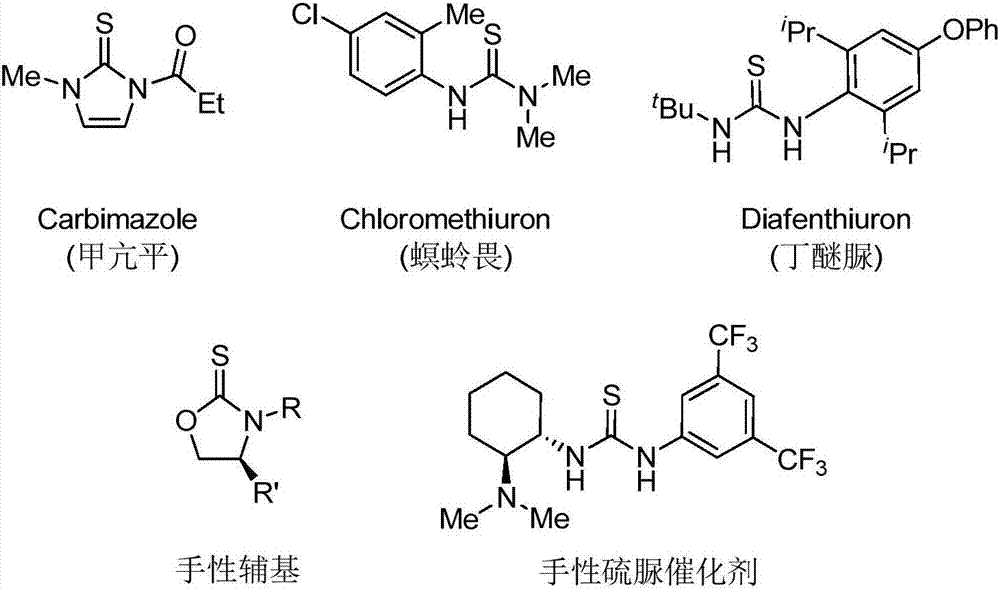
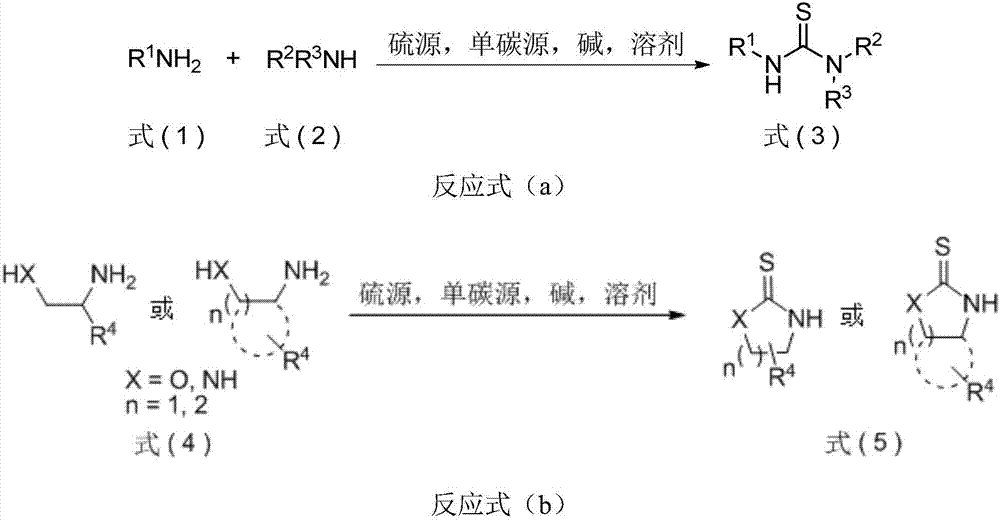
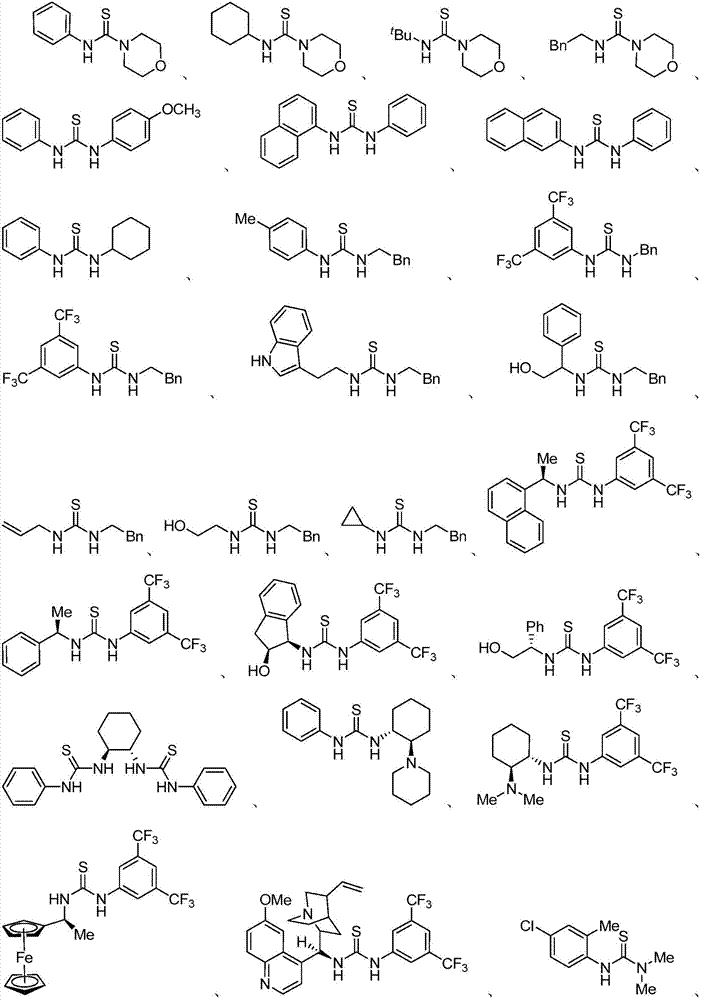
Thiourea and oxazolidine thione compounds and synthesizing method and application thereof
A technology of oxazolidinethione and synthesis method, applied in organic chemistry methods, chemical instruments and methods, organic chemistry and other directions, can solve the problems of high toxicity, low atomic economy, bad odor and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The method for synthesizing thiourea and oxazolidinethione compounds of the present invention comprises the following steps: adding an amine compound (X mmol), a base (Y mmol), a solvent (V mL), and chloroform (Z mmol) in a reaction vessel, The reaction system was stirred at 55°C for 4 hours; after that, the reaction system was cooled to room temperature, amine compound (W mmol), sulfur powder (U mmol), and alkali (Hmmol) were added, and the reaction system was heated from room temperature to 55°C. conduct. Monitor the progress of the reaction. After the reaction was completed, at room temperature, saturated ammonium chloride solution (PmL) was added to the system to quench the reaction, extracted three times with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain target product.
[0059] The method for synthesizing thiourea and oxazolidinethione compounds of the present in...
Embodiment 1
[0061] Synthesis of compound 3k:
[0062]
[0063] In the reaction tube, add substrate 1k (0.2mmol, 45.8mg), potassium tert-butoxide (1.2mmol, 134.7mg), 1,4-dioxane / tert-butanol (0.4 / 0.4mL), chloroform in sequence (2 mmol, 238.8 mg), the reaction system was stirred at 55°C for 2 hours. Then, the reaction was cooled to room temperature, and sulfur powder (0.6mmol, 19.2mg), potassium tert-butoxide (0.4mmol, 44.9mg), 2k (0.24mmol, 29.0mg) were added successively, the temperature was raised to 55° C. and the reaction was continued for 8 hours. Monitoring reaction process. After the reaction was completed, 3 mL of saturated ammonium chloride solution was added to the system to quench the reaction at room temperature, extracted three times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain the compound 3k (36.8 mg, 47%).
Embodiment 2
[0065] Synthesis of compound 3k:
[0066]
[0067]In the reaction tube, add substrate 1k (0.6mmol, 137.4mg), potassium tert-butoxide (1.2mmol, 134.7mg), 1,4-dioxane / tert-butanol (0.4 / 0.4mL), chloroform in sequence (2 mmol, 238.8 mg), the reaction system was stirred at 55°C for 2 hours. Then, the reaction was cooled to room temperature, and sulfur powder (0.6mmol, 19.2mg), potassium tert-butoxide (0.4mmol, 44.9mg), 2k (0.2mmol, 24.2mg) were added successively, and the temperature was raised to 55° C. to continue the reaction for 8 hours, monitoring reaction process. After the reaction was completed, 3 mL of saturated ammonium chloride solution was added to the system to quench the reaction at room temperature, extracted three times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, concentrated, and separated by column chromatography to obtain the compound 3k (55.7 mg, 71%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com