Axial chiral styrene tertiary amine thiourea catalyst as well as preparation method and application thereof
A styrene-based, tertiary thiourea technology, applied in the direction of organic chemical methods, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of poor catalytic effect and poor control of stereoselectivity, and achieve good stereo Selectivity, excellent catalytic effect, suitable for large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
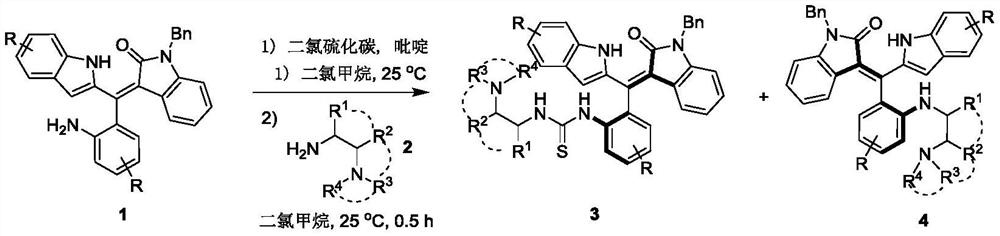
[0027] The synthetic route of the present embodiment is as follows:
[0028]
[0029] The specific steps are
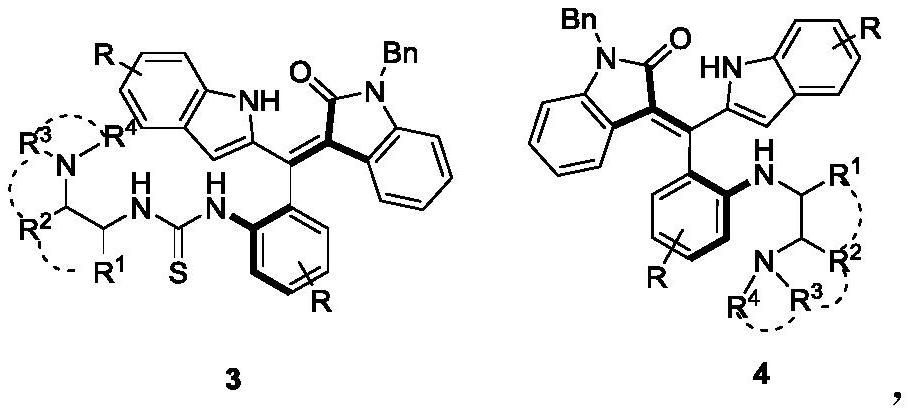
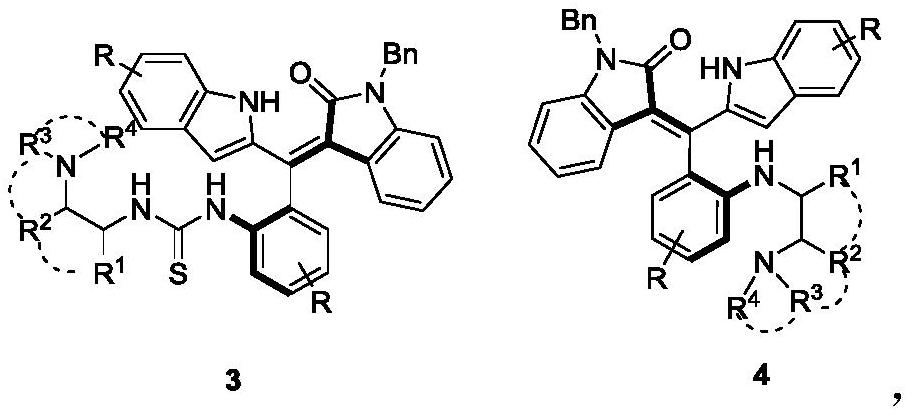
[0030] Add 0.2mmol of the compound of formula 1 to 2ml of dichloromethane, then add 0.3mmol of pyridine and 0.24mmol of carbon dichloride in turn, react at 25°C for 1 hour, follow the reaction to the end by TLC, add 2ml of dichloromethane after concentration , 0.3mmol(S)-N 1 ,N 1 , 3-trimethylbutane-1,2-diamine, reacted at 25°C for 0.5 hours, TLC tracked the reaction to the end, concentrated and passed through silica gel column chromatography (dichloromethane with a volume ratio of 50:1 was used to remove the agent) / methanol mixture) to separate compounds of formula 3a and formula 4a.
[0031] The structural characterization data of product 3a are as follows:
[0032] yield:28% (34.3mg); yellow solid; m.p.81.6–81.9℃; [α] D 20 =+43.8(c 0.03, acetone); 1 H NMR(400MHz,DMSO)δ13.94(s,1H),8.50(s,1H),8.12–8.04(m,1H),7.61–7.56(m,2H),7.49–7.43(m,3H), 7.41–7.33(m,4H...
Embodiment 5
[0041] Add 0.1 mmol of p-methoxyphenyl-substituted o-methylenebenzoquinone (formula 5) and 0.006 mmol of catalyst 3d (the product in Example 4) to 1 ml of toluene, and lower the temperature to -50°C. Add 0.1 mmol of malononitrile, react at -50°C for 4 hours, follow the reaction to the end by TLC, concentrate and separate by silica gel column chromatography (the deagent uses a dichloromethane / methanol mixture with a volume ratio of 50:1), That is, the compound of formula 6 is obtained.
[0042] The structural characterization data of product 6 are as follows:
[0043] Yield: 90% (49.0mg); Yellow solid, m.p.102-103°C; [α] D 20 = -39.5(c 0.35, Acetone); 1 H NMR (400MHz, CDCl 3 )δ7.10(d, J=8.6Hz, 2H), 6.85(d, J=8.6Hz, 2H), 6.50(s, 1H), 6.34(s, 1H), 5.92(s, 1H), 5.89( s,1H),4.58(s,1H),4.54(s,2H),3.78(s,3H); 13 C NMR (100MHz, CDCl 3 )δ159.0,158.9,147.2,144.9,143.0,136.9,129.0,120.0,115.3,114.3,107.9,101.7,97.9,60.9,55.4,40.5; IR:2924,2189,1684,1507,1371,135,7cm -1 ;ESI FTMS ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com