A process for producing enzalutamide
A kind of enzalutamide, suitable technology, be applied in the field of preparation medicine enzalutamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
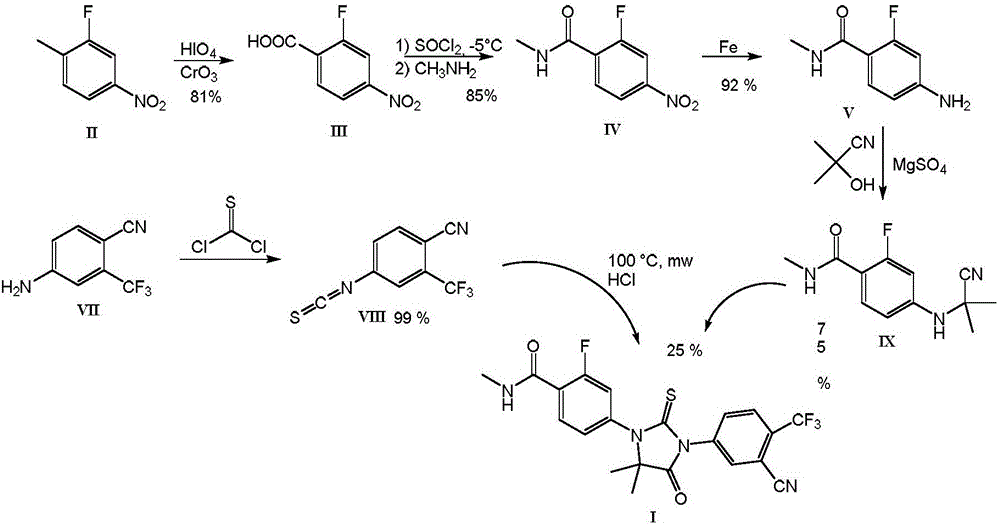
[0036] 2-fluoro-4-nitro-N-methylbenzamide of formula IV.
[0037]
[0038] Add 2000 ml of isopropyl acetate, 10 ml of DMF to 250 g (1.35 mol) of 2-fluoro-4-nitrobenzoic acid of formula III in a 5000-ml reactor, heat the mixture to 60° C., and place the container with Flush well with nitrogen. Then, 193 g (1.62 mol) of thionyl chloride were added dropwise within 50 minutes, and the mixture was further stirred at 60° C. overnight. The next day, the mixture was cooled to -10 °C, the turbidity was filtered through a folded filter, and the obtained clear solution of 2-fluoro-4-nitrobenzoyl chloride was transferred to a bottle and kept under an inert nitrogen atmosphere .
[0039] 500 ml of isopropyl acetate, 524 g of 40% aqueous methylamine solution were charged into the reaction vessel, and the mixture was cooled to 0°C. To this mixture was added dropwise a solution of 2-fluoro-4-nitrobenzoyl chloride over 4 hours, and the resulting mixture was stirred for a further 1 hour. ...
Embodiment 2
[0042] 4-Amino-2-fluoro-N-methylbenzamide hydrochloride of formula V.
[0043]
[0044] 40.0 g (0.202 mol) of 2-fluoro-4-nitro-N-methylbenzamide of the formula IV were charged into a 1500 ml autoclave, and 500 ml of methanol and 0.50 g of 10% by weight palladium on activated carbon were added. The mixture was hydrogenated at a pressure of 600 kPa and a temperature of 50° C. for 1 hour, then the catalyst was removed by filtration through a bed of celite and the solvent was evaporated to dryness. The evaporated product (35.6 g) was dissolved in 180 ml of hot ethanol and 21 g of concentrated (35%) hydrochloric acid (0.202 mmol) were added. After inoculation and cooling, the solid product that separated was aspirated and washed with ethanol. 29.3 g (71% by weight) of the product of the formula V are obtained in the form of white crystals with a melting point of 222° C. to 228° C. (dec.). HPLC purity: 99.89%. 1 H NMR (DMSO): δ (ppm): 2.75 (d, 3H), 6.80 (m, 2H), 7.57 (t, 1H), ...
Embodiment 3
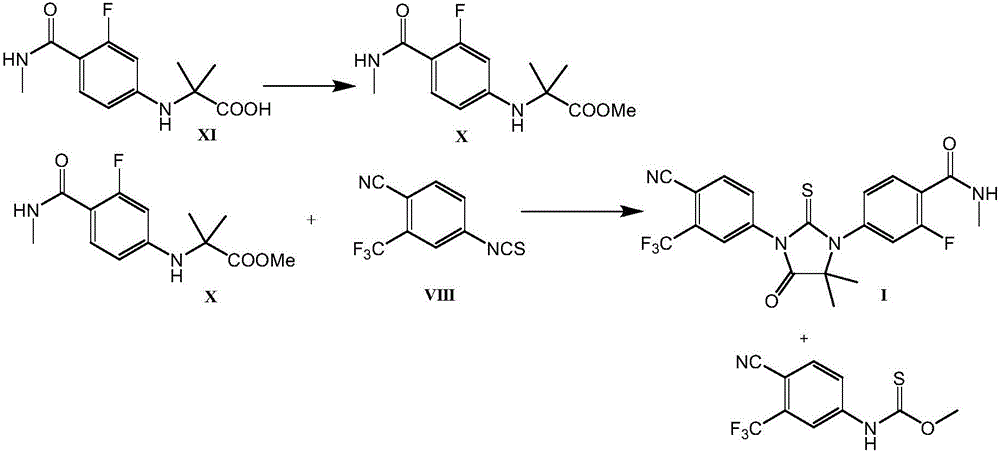
[0046] 2-[3-fluoro-4-(methylcarbamoyl)phenylamino]-2-methylpropionic acid of formula XI.
[0047]
[0048] 50.0 g (0.244 mol) of 4-amino-2-fluoro-N-methylbenzamide hydrochloride of formula V were charged into a 500 ml container equipped with an anchor stirrer, 50 ml of dimethylacetamide was added, and The mixture was heated to 70°C. Then, 68.0 g (0.672 mol) of triethylamine was added, and the mixture was stirred for 60 minutes. Thereafter, the temperature was raised to 100° C., and a preheated solution of 54.3 g (0.325 mol) of 2-bromo-2-methylpropionic acid in 25 ml of dimethylacetamide was added dropwise to the mixture during 10 minutes . The mixture was stirred at 100°C for a further 1 1 / 2 hours, then it was cooled to 40°C and 125ml of water and a solution of 40g of citric acid in 100ml of water were added. The mixture was seeded with product, cooled to 20°C, and stirred overnight. The isolated product was suctioned on a Buchner funnel, washed with a considerable amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com