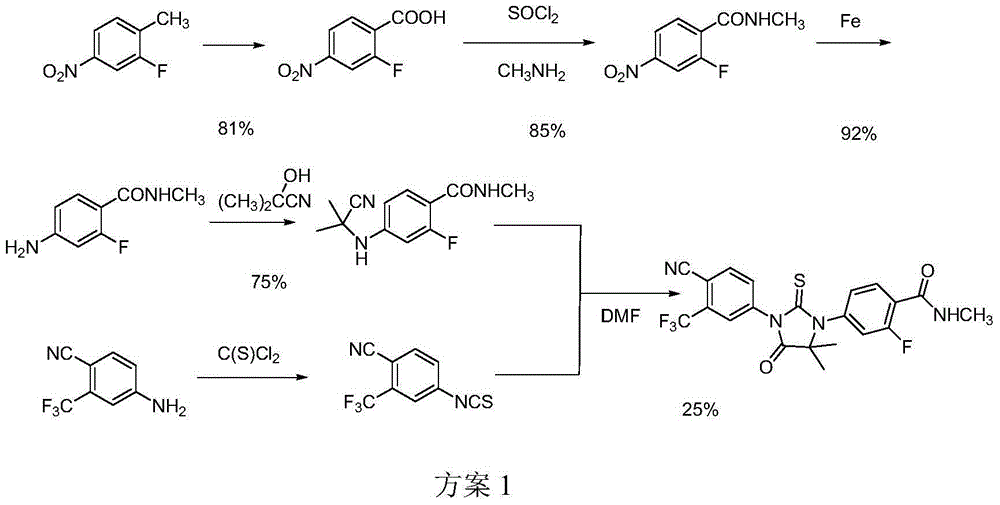
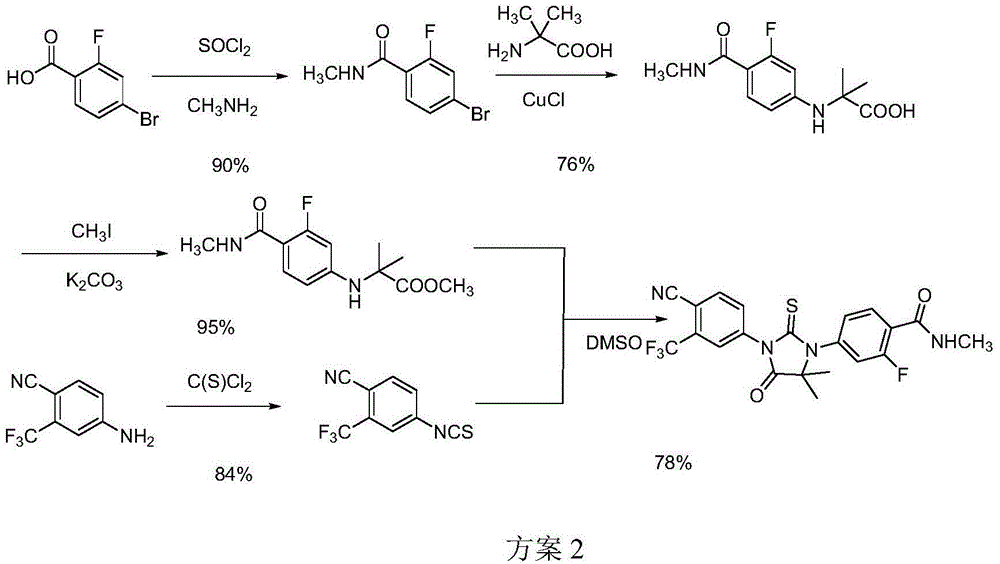
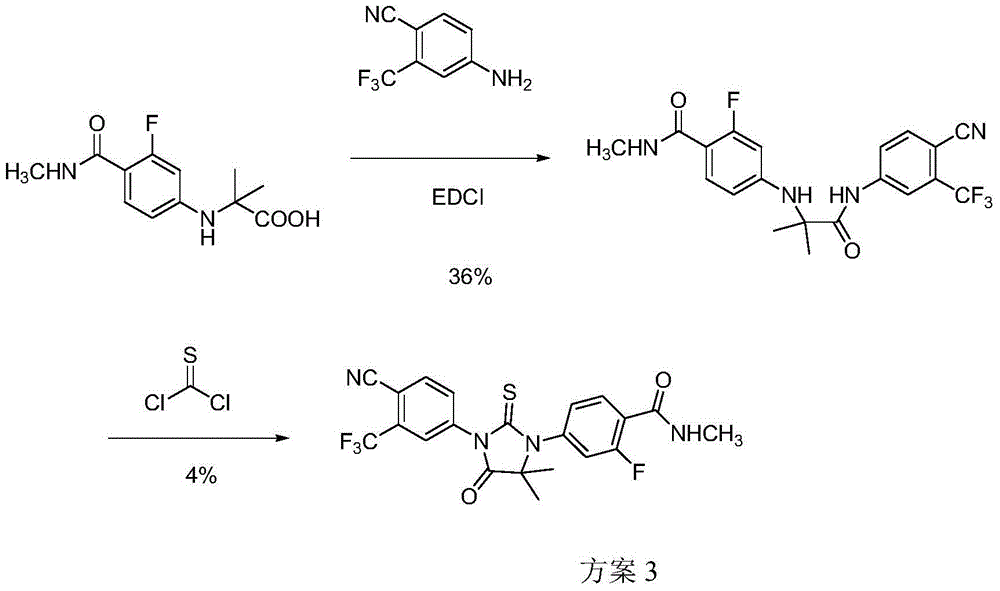
Preparation method of enzalutamide
A technology of enzalutamide and methylamine, which is applied in the field of prostate cancer drugs, can solve the problems of low total yield, low yield, labor protection and environmental hazards, and achieve convenient and simple post-treatment, mild reaction conditions and low production cost. Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of 2-(3-fluoro-4-(methoxyformyl)phenylamino)-2-methylpropanoic acid (C)
[0057]
[0058] Add 4-bromo-2-fluoro-benzoic acid methyl ester (A, 100g), 2-aminoisobutyric acid (66g, 1.5 equivalents), cuprous chloride (8.4g, 0.2 equivalents) into a 1L four-neck flask )﹑Potassium carbonate (148g, 2.5 equivalents)﹑DMF (700ml)﹑H 2 O (5.0ml) was heated to 105°C for 14h under nitrogen protection, cooled to room temperature, and isopropyl acetate (600ml) and H 2 O (1.2L), stirred, separated, took the water layer, added 1M citric acid aqueous solution to adjust pH = 4, precipitated solid, cooled to below 5°C, filtered with suction, dried to obtain the product 2-(3-fluoro -4-(Methoxyformyl)phenylamino)-2-methylpropanoic acid (C, 85.9 g, yield 78.5%). MSm / z256[M+H] + ; 1 HNMR (400Hz, DMSO-d 6 )δ7.81-7.79(d,J=8.0Hz,1H),7.52(s,1H),7.47(br,1H),7.40-7.38(d,J=8.0Hz,1H),3.77(s,3H ), 1.59(s,6H).
Embodiment 2
[0060] Preparation of methyl 2-(3-fluoro-4-(methoxyformyl)phenylamino)-2-methylpropanoate (D)
[0061]
[0062] Add 2-(3-fluoro-4-(methoxyformyl)phenylamino)-2-methylpropionic acid (C, 100g), MeOH (1.0L), DMF (3.0ml) into a 2L four-neck flask ) after cooling to below 10°C, drop in SOCl 2 (30ml, 1.05 equivalents), reflux reaction for 12h after the dropwise addition, after TCL monitors the reaction, evaporate the solvent to dryness, add H 2 O (400ml) and EtOAc (400ml), stirred, added dropwise sodium carbonate aqueous solution to adjust pH = 8, separated, took the organic layer, evaporated to dryness, added petroleum ether (400ml) and stirred and washed to obtain the white solid product 2-(3 -Methyl fluoro-4-(methoxyformyl)phenylamino)-2-methylpropanoate (D, 101.5 g, yield 96.2%). MSm / z270[M+H] + ; 1 HNMR (400Hz, DMSO-d 6 )δ7.80-7.78(d,J=8.0Hz,1H),7.53(s,1H),7.48(br,1H),7.40-7.38(d,J=8.0Hz,1H),3.77(s,3H ), 3.69(s,3H), 1.58(s,6H).
Embodiment 3
[0064] Preparation of 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl) -2-Fluoro-benzoic acid methyl ester (F)
[0065]
[0066] Add 2-(3-fluoro-4-(methoxyformyl)benzylamino)-2-methylpropionic acid methyl ester (D, 50g)﹑4-isothiocyanato-2 into a 500ml four-necked bottle -(Trifluoromethyl)benzonitrile (E, 84.7g, 2eq), DMSO (50ml) and isopropyl acetate (100ml). Heat the reaction solution to 90°C for 20h, cool to room temperature, add MeOH (15ml), heat to 70°C for 40min, cool to room temperature, add isopropyl acetate (600ml)﹑HO 2 O (300ml) and IPA (100ml), stirred and separated, evaporated the organic phase to remove the solvent to about 450ml, added IPA (1.2L) and heated to 80°C to dissolve completely, then cooled to 10°C, a white solid was precipitated, filtered with suction , dried to give 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidine-1 -yl)-2-fluoro-benzoic acid methyl ester (F, 71.7g, yield 83.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com