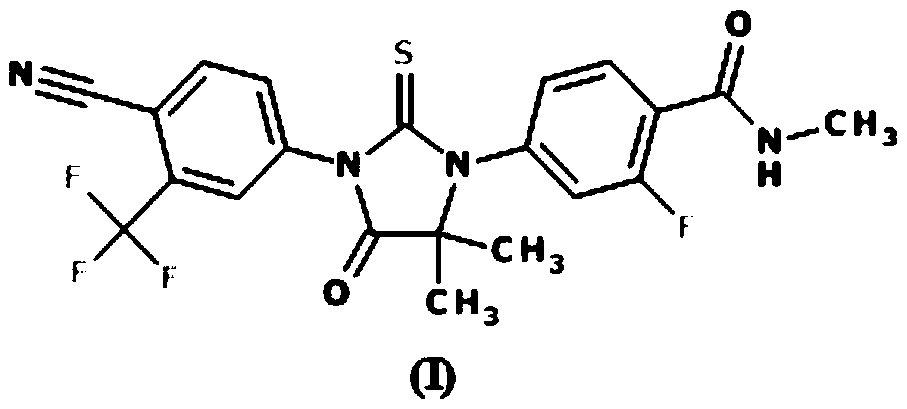
Process for preparation of enzalutamide using novel intermediate
A technology of enzalutamide and tertiary amine base, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
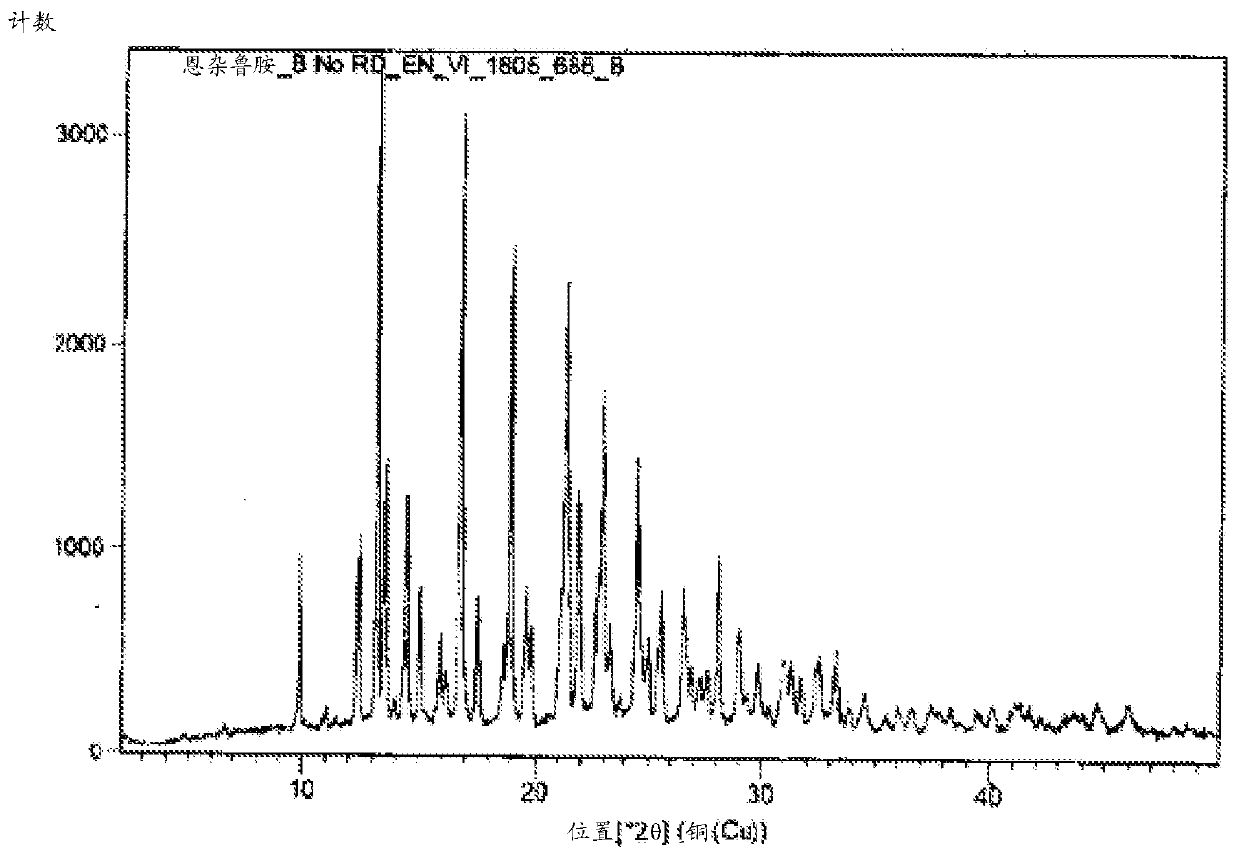
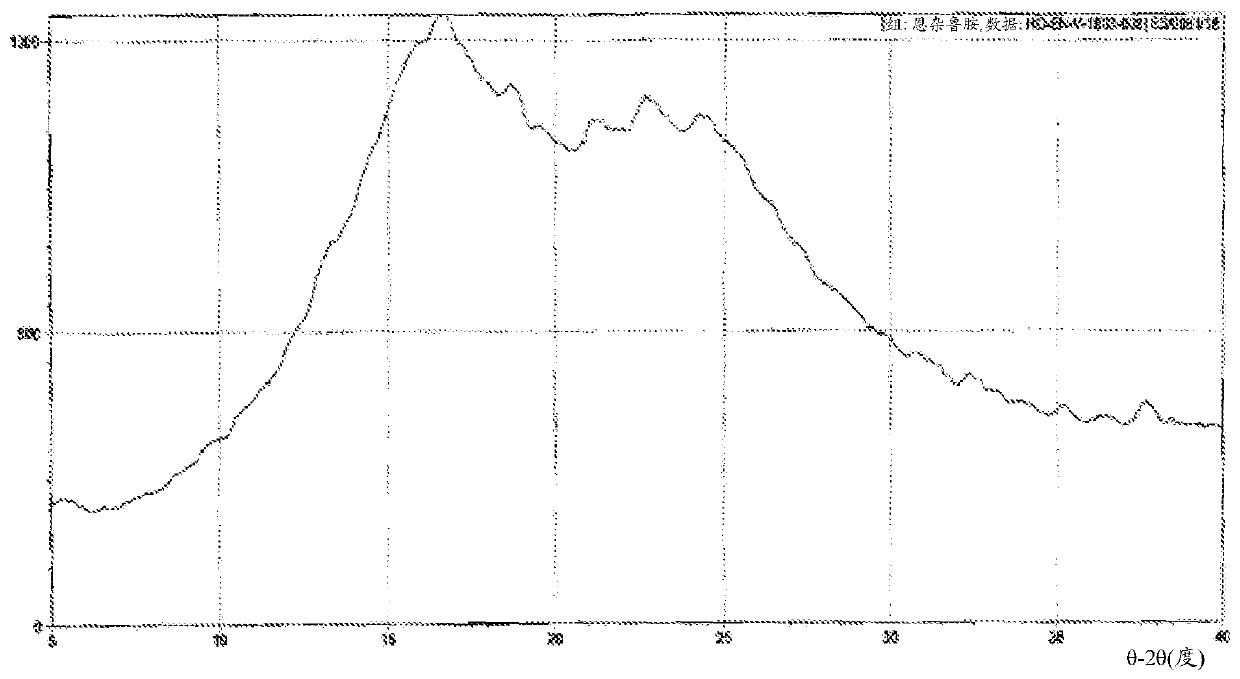
Image
Examples
Embodiment 1
[0155] Preparation of 4-bromo-2-fluoro-N-methylbenzamide (Formula D)
[0156] Dimethylformamide (1 ml) was added to a mixture of 4-bromo-2-fluorobenzoic acid (100 g) and toluene (200 ml) with stirring at a temperature of about 30°C to 35°C. The temperature of the reaction mixture was raised to 65°C to 70°C. Thionyl chloride (100 g) was slowly added to the mass at 65°C to 70°C. The mass was stirred for 1 hour to 2 hours to obtain a clear solution. The mass was cooled to 10°C to 15°C. This material was added slowly to a 40% solution of monomethylamine (281 ml) precooled to 15°C. The mixture was stirred at 10°C to 15°C for 1 hour. The material was filtered under a vacuum of 50 to 60 Torr. The reaction mass was washed with toluene (50ml) and then with water (100ml). The product was dried in a hot air oven at a temperature of about 60°C to about 65°C to obtain a dry weight (100 g) with a purity of 99.44%.
Embodiment 2
[0158] Preparation of 2-[3-fluoro-4-(methylcarbamoyl)anilino]-2-methyl-propionic acid (formula III)
[0159] Copper chloride (12.8 gm) was added to N,N'-dimethylformamide (600 ml) at 75°C to 80°C under argon atmosphere. 4-Bromo-2-fluoro-N-methylbenzamide (100 g) obtained in Example 1 was added to the mixture, and then N,N-dimethylaniline (8.35 gm) was added. The reaction mixture was stirred for 15 minutes to 20 minutes. 2-Aminoisobutyric acid (67 gm) was added and the mixture was stirred again at 75°C to 80°C for 15 minutes to 20 minutes. Powdered potassium carbonate (148gm) was added and stirred for 15 minutes. Water (10ml) was added, the temperature of the mass was raised to 110°C and the mass was stirred for 4-5 hours. The mass was cooled to 30°C to 35°C and filtered. The material was filtered and washed with dimethylformamide. The dimethylformamide was distilled off under vacuum and the mass was cooled. Water (700ml) was added to the mass and the pH was adjusted to 1...
Embodiment 3
[0162] Preparation of N-[4-cyano-3-(trifluoromethyl)phenyl]carbamoic acid, 1,8-BDU complex via [4-cyano-3-(trifluoromethyl)phenyl] ] Methyl carbamate
[0163] I) [4-cyano-3-(trifluoromethyl) phenyl] aminodithioformic acid, preparation of 1,8-BDU complex (formula V)
[0164] 4-Amino-2-(trifluoromethyl)benzonitrile (100 g) was added to acetone (200 ml) at 25°C to 30°C. The reaction mixture was cooled to 15°C to 20°C. 1,8-DBU (245 g) was slowly added to the reaction mass at a temperature of about 15°C to about 20°C, and carbon disulfide (211 gm) was added dropwise over 30 minutes at a temperature of about 15°C to about 20°C. The reaction mass was maintained at 25°C to 30°C for 3 to 4 hours, the product precipitated out, after which the mixture was stirred for 1 hour and the reaction mass was quenched in DM water (3 L). The mass is maintained at a temperature of about 25°C to about 30°C for 30 minutes. The reaction mass was filtered under vacuum and the wet cake was washed wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com