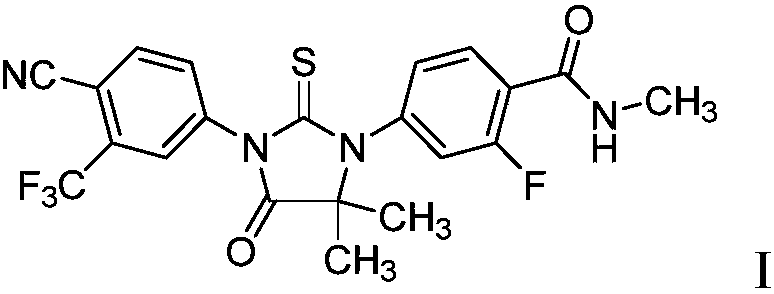
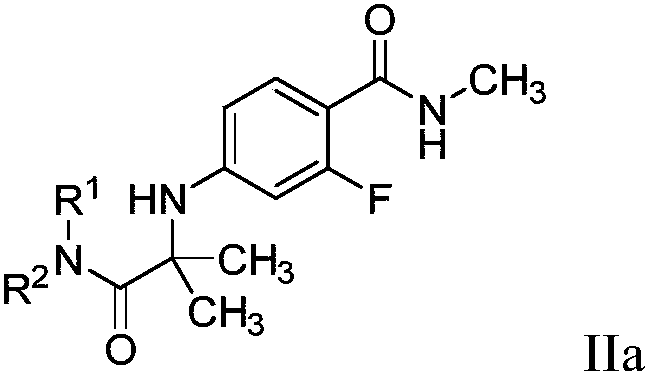
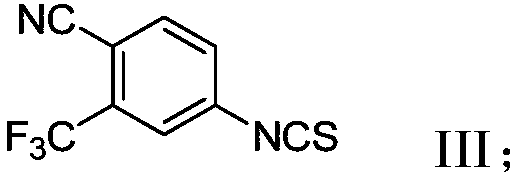
A novel process for preparing enzalutamide
A technology of impurities and primary amines, applied in the new field of preparation of enzalutamide, can solve problems such as limited synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Preparation of diamide 14
[0108]
[0109] A four-neck round bottom flask was equipped with a mechanical stirrer and a thermometer. At 20-30°C, under nitrogen, add compound 8 (10g, 39.39mmol, 1 equivalent), EDCI (7.33g, 47.2mmol, 1.2 equivalents), HOBt (7.20g, 47.3mmol, 1.2 equivalents) and DMF (50 mL, 5 vol). The mixture was stirred at 20-30 °C for 5 min, then the EtNH 2 (2M in THF, 49 mL, 98 mmol, 2.5 eq) was added to the reaction flask. The reaction mixture was stirred at 20-30°C for 15 hours. After the reaction was complete, EtOAc (100 mL, 10 vol) and saturated NH 4 Cl(aq) (100 mL, 10 vol), stirring was continued for 5 minutes. The resulting mixture was filtered and the filtrate was added to H 2 O (50mL). The organic portion was withdrawn and the aqueous layer was washed with EtOAc (3 x 50 mL, 5 vol). The combined organic fractions were concentrated to dryness to afford about 7 g of crude compound 14 as a yellow oil. To the yellow oil was added EtOAc (5...
Embodiment 2
[0111] Cyclization reaction: amide method
[0112]
[0113] A four-neck round bottom flask was equipped with a mechanical stirrer and a thermometer. At 20-30°C, in nitrogen, compound 14 (0.5g, 2mmol, 1 equivalent), compound 4 (0.89g, 3.9mmol, 2.2 equivalents) were added to the flask, Molecular sieves (0.5 g, 1 wt) and MeCN (2 mL, 4 vol). The reaction mixture was stirred at 20-30°C to remove water. After 16 hours, the reaction mixture was heated to 60-70°C for 24 hours. Another amount of compound 4 (1.29 g, 5.66 mmol, 3.2 equiv, twice) was added, and stirring was continued for 24 hours. After the reaction was complete, EtOAc (10 mL, 20 vol) was added to the reaction mixture, H 2 O (10 mL, 20 vol) and saturated NaCl(aq) (5 mL, 10 vol), stirring was continued for 5 minutes. The mixture was filtered and the phases were separated. The separated aqueous layer was extracted with EtOAc (50 mL, 5 vol). The combined organic fractions were concentrated to near dryness to afford ...
Embodiment 3
[0115] Cyclization Reaction: The Acid Method
[0116]
[0117] A four-neck round bottom flask was equipped with a mechanical stirrer and a thermometer. Compound 8 (5 g, 19.66 mmol, 1 eq), THF (25 mL, 5 vol) and 2N NaOH(aq) (14.8 mL, 29.6 mmol, 1.5 eq) were added to the flask under nitrogen at 20-30 °C. The mixture was stirred at 20-30°C for 30 minutes. Compound 4 (13.5 g, 58.98 mmol, 3.6 equiv) was added at 20-30°C in three equal portions every 5-6 hours. After the reaction was complete, EtOAc (25 mL, 5 vol) and H 2 O (10 mL, 2 vol). The mixture was stirred for 5 minutes, then the phases were separated. The separated aqueous portion was extracted with EtOAc (25 mL, 5 vol). The combined organic fractions were concentrated to near dryness to afford 10.5 g of crude enzalutamide as a yellow oil. IPA (50 mL, 10 vol) was added to the yellow oil, and the mixture was heated to 50-60 °C to obtain a homogeneous solution. The mixture was cooled to 20-30°C and stirred for 1 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com