Process for producing enzalutamide crystal form
A technology of enzalutamide and a manufacturing method, applied in the field of manufacturing enzalutamide crystalline form, can solve problems such as unclear details
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] (Example 1) Synthesis of 4-cyano-3-trifluoromethylphenyl isothiocyanate
[0088] Isopropyl acetate (IPAc) (20 mL) / water solution (56 mL) of thiophosgene (14.9 g) was prepared, and 4-cyano-3-trifluoromethylaniline (20 g) was dissolved therein dropwise over 30 minutes. IPAc solution (90 mL). The internal temperature was 4°C. After stirring for 5 minutes at an internal temperature of 4° C. for 5 minutes, the mixture was left to stand for 30 minutes or more to separate the water layer. The obtained organic layer was concentrated under reduced pressure, n-heptane was added to the concentrated residue, and further concentrated under reduced pressure to 80 mL or less. IPAc (1 mL) was added to the obtained concentrated residue, stirred at an internal temperature of 40°C for 5 minutes, then seed crystals (10 mg) were added at 25°C, stirred for 1 hour, stirred at an internal temperature of 4°C, and filtered to obtain 4-Cyano-3-trifluoromethylphenylisothiocyanate (22.1 g) was o...
Embodiment 2
[0089] (Embodiment 2) Synthesis of Enzalutamide Type A Crystal
[0090] In a nitrogen atmosphere, 2-(3-fluoro-4-methylcarbamoyl-phenylamino)-2-methylpropionic acid methyl ester (33.0g) and the 4-cyano- 3-Trifluoromethylphenylisothiocyanate (56.1g) was dissolved in a mixed solvent of dimethylsulfoxide (DMSO) (33mL) / IPAc (66mL), and the temperature was raised to an internal temperature of 75-85°C. The temperature was stirred for more than 12 hours. After the reaction, methanol (4.95 mL) was added dropwise at 55-80° C., and stirred at this temperature for 60-90 minutes. Then, it cooled to 15-25 degreeC, diluted with IPAc (198 mL) and pure water (99 mL), stirred at this temperature for 10-30 minutes, and left still for 30-45 minutes. 2-Propanol (IPA) (49.5 mL) was slowly added dropwise at an internal temperature of 15 to 25° C. to break the emulsion. The obtained organic layer was separated, and the line was washed with IPAc (15 mL).
[0091] The organic layer after liquid sep...
reference example 1-1
[0100] (Reference Example 1-1) Synthesis of Enzalutamide Type B Crystal
[0101] In a nitrogen atmosphere, a solution of purified crystals (10.0 g) of enzalutamide type A crystals in IPA (80 mL) was stirred at room temperature. At 20 to 30° C., a seed crystal (10.2 mg=0.1% by mass) of enzalutamide type B crystal was added, and stirred at this temperature for 4 days. After stirring, the precipitated crystals were collected by filtration. Then, it was washed with IPA (10 mL), and dried under reduced pressure at 55° C. for about 4 hours to obtain 10.3 g of enzalutamide type B crystals of 1 / 2 solvate of IPA. The yield was 96.2%.
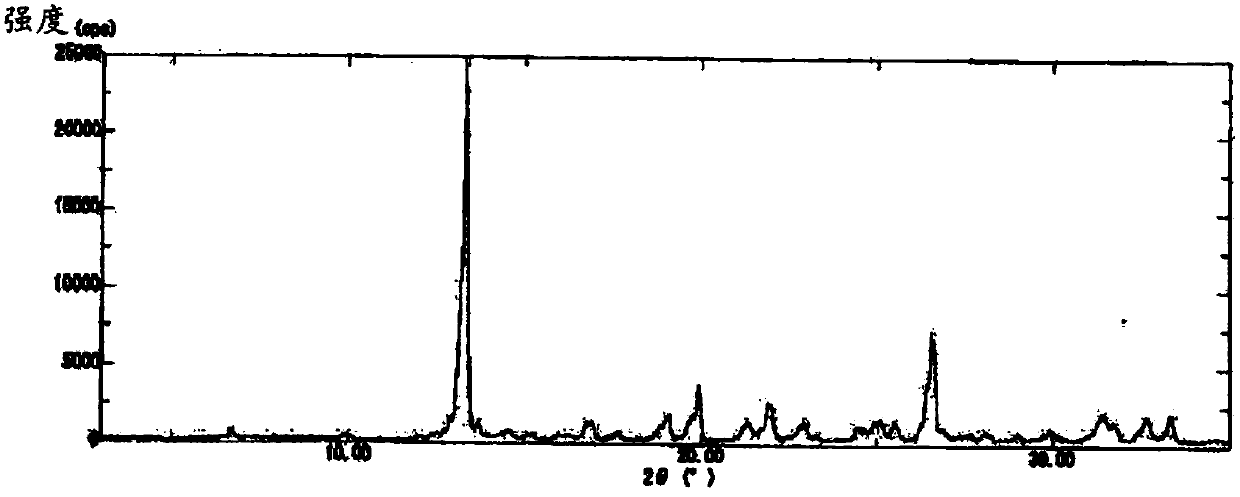
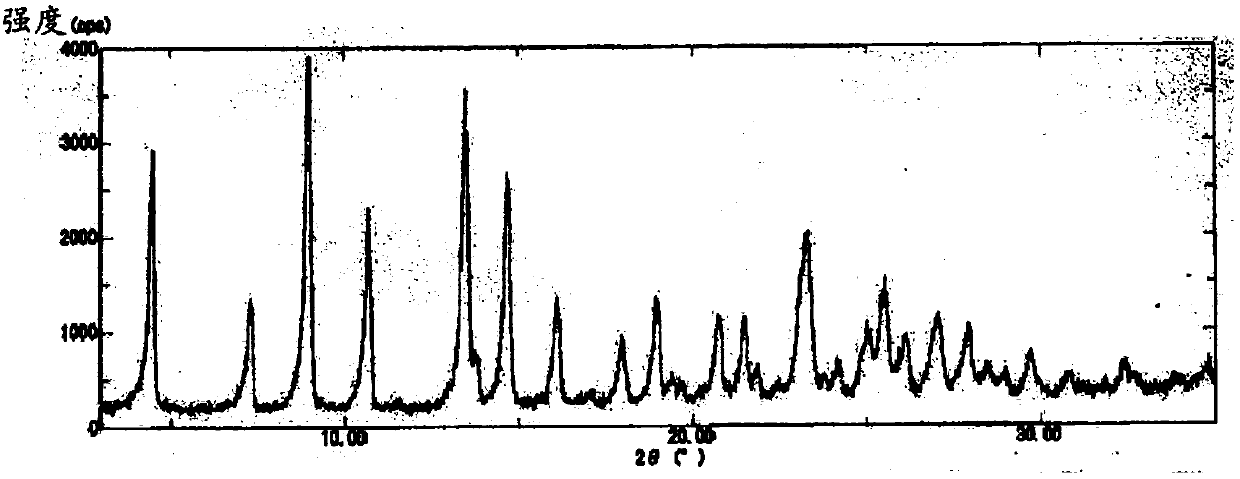
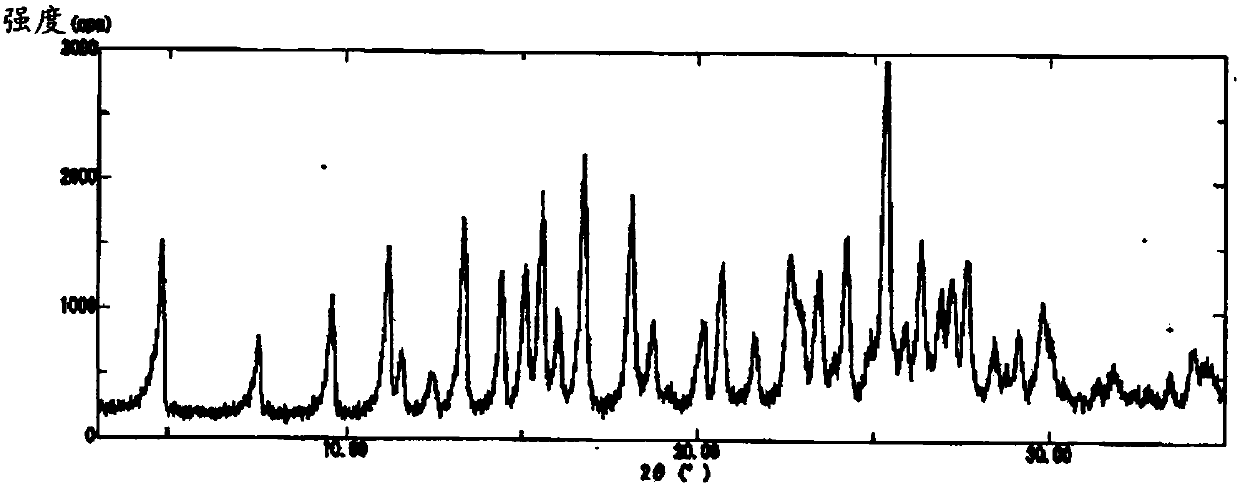
[0102] The obtained B-type crystals were respectively 1 The results of H-NMR are shown below, the results of elemental analysis are shown in Table 2, and the results of XRD measurement are shown in figure 2 , and the 2θ values of the peak tops in the XRD pattern are shown below. In addition, in DSC analysis, endothermic peaks were observed from a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com