A kind of enzalutamide soft capsule and preparation method thereof
A technology of enzalutamide and soft capsules, which is applied in the field of medicine and can solve the problems of low bioavailability, incompleteness, and low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
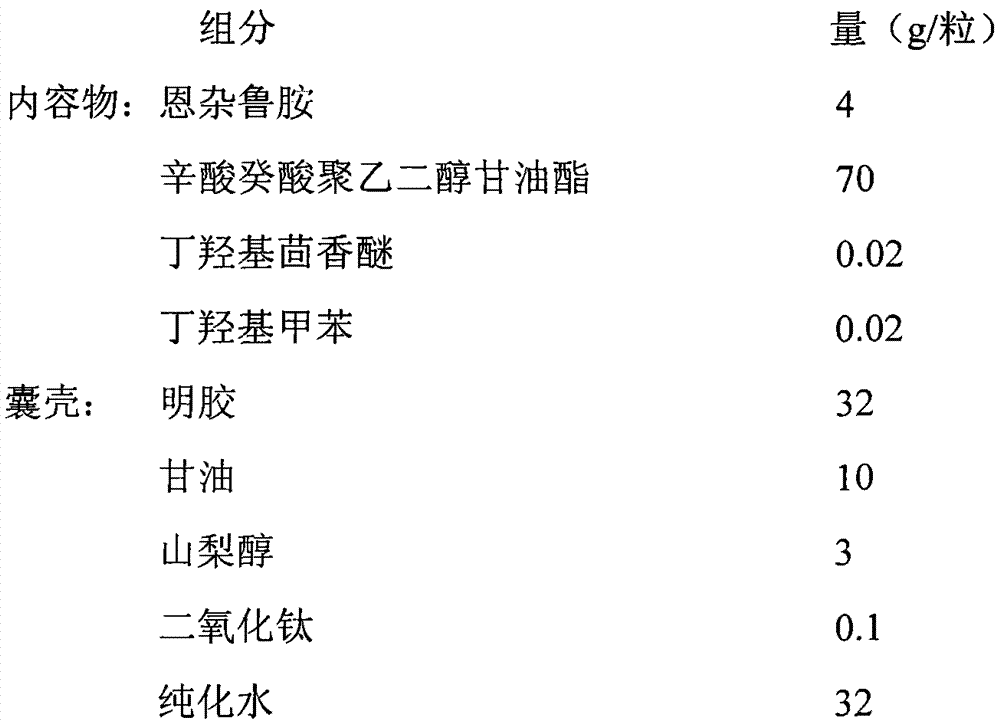
[0019]
[0020]
[0021] Preparation:
[0022] (1) Preparation of the contents: heat caprylic capric acid macrogol glyceride to 60-70°C, add butylated hydroxyanisole, butylated hydroxytoluene and enzalutamide, and stir until the raw and auxiliary materials are completely dissolved;
[0023] (2) Preparation of capsule shell glue: add purified water, glycerin and sorbitol into the glue tank and heat to 60-80°C, stir until the solution is clear; add titanium dioxide, stir until the gelatin is evenly dispersed; add gelatin, then add prescription amount of gelatin. Turn on the vacuum pump, degas for ≥3 hours under the condition of ≤-0.08Mpa, keep warm at 70±5°C, and set aside;
[0024] (3) Preparation of soft capsules: take the prepared contents and capsule shells, make soft capsules by pressing, shape, dry, wash pills, pick pills, and pack.
Embodiment 2
[0026]
[0027] Preparation:
[0028] (1) Preparation of the contents: heat caprylic capric acid macrogol glyceride to 60-70°C, add butylated hydroxyanisole, butylated hydroxytoluene and enzalutamide, and stir until the raw and auxiliary materials are completely dissolved;
[0029] (2) Preparation of capsule shell glue: add purified water, glycerin and sorbitol into the glue tank and heat to 60-80°C, stir until the solution is clear; add titanium dioxide, stir until the gelatin is evenly dispersed; add gelatin, then add prescription amount of gelatin. Turn on the vacuum pump, degas for ≥3 hours under the condition of ≤-0.08Mpa, keep warm at 70±5°C, and set aside;
[0030] (3) Preparation of soft capsules: take the prepared contents and capsule shells, make soft capsules by pressing, shape, dry, wash pills, pick pills, and pack.
Embodiment 3
[0032]
[0033] Preparation:
[0034] (1) Preparation of the contents: heat caprylic capric acid macrogol glyceride to 60-70°C, add butylated hydroxyanisole, butylated hydroxytoluene and enzalutamide, and stir until the raw and auxiliary materials are completely dissolved;
[0035] (2) Preparation of capsule shell glue: add purified water, glycerin and sorbitol into the glue tank and heat to 60-80°C, stir until the solution is clear; add titanium dioxide, stir until the gelatin is evenly dispersed; add gelatin, then add prescription amount of gelatin. Turn on the vacuum pump, degas for ≥3 hours under the condition of ≤-0.08Mpa, keep warm at 70±5°C, and set aside;
[0036] (3) Preparation of soft capsules: take the prepared contents and capsule shells, make soft capsules by pressing, shape, dry, wash pills, pick pills, and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com