A kind of preparation method of enzalutamide
A technology based on enzalutamide and phenylamine, which is applied in the field of preparation of enzalutamide, can solve the problems of harsh reaction conditions, harm to the environment, and low yield of large-scale production, so as to increase yield and reduce environmental pollution , Overcome the effect of harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of enzalutamide:
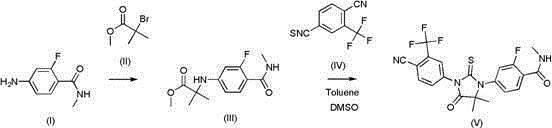
[0039] Add 20g (0.119mol) of 4-amino-2-fluoro-N-methylbenzamide (I) and 86.2g (0.476mol) of methyl 2-bromoisobutyrate (II) into a 250ml reaction flask, then Add 50.5g (0.476mol) sodium carbonate to the bottle, heat to 130°C, stir and react for 16 hours, monitor the reaction progress by HPLC, add 150ml water to the reaction solution after the reaction, then add 150ml ethyl acetate to extract, use the same amount The ethyl acetate was extracted three times, the ethyl acetate layers were combined, washed once with 100ml of saturated brine, the ethyl acetate was dried with anhydrous sodium sulfate, filtered, and the ethyl acetate was spin-dried to obtain a yellow solid, and 100ml of methanol was added to the yellow solid Slurry for 5 hours, filter, and dry to obtain 27.2 g of white solid 2-(3-fluoro-4-(methylaminocarboxamide) phenylamino)-2-methylpropanoic acid methyl ester (III), HPLC purity 99.5% , Yield 85% (based on comp...
Embodiment 2
[0041] Embodiment 2: the preparation of enzalutamide:
[0042] Add 200g (1.19mol) of 4-amino-2-fluoro-N-methylbenzamide and 862g (4.76mol) of methyl 2-bromoisobutyrate into a 5L reaction flask, and then add 614g (4.76 mol) diisopropylethylamine, heated to 130°C and stirred for 16 hours. The reaction process was monitored by HPLC. After the reaction, 1500ml of water was added to the reaction liquid, and then 1500ml of ethyl acetate was added for extraction. Extract three times, combine the ethyl acetate layers, wash once with 100ml saturated brine, dry the ethyl acetate with anhydrous sodium sulfate, filter, spin dry the ethyl acetate to obtain a yellow solid, add 1000ml methanol to the yellow solid for 5 hours, filter Dry to obtain white solid 2-(3-fluoro-4-(methylaminocarboxamide) phenylamino)-2-methylpropanoic acid methyl ester (III), 258g, HPLC purity 99.3%, yield 81% ( Calculated as compound I).
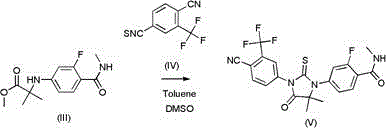
[0043] Add 100g (0.373mol) 2-(3-fluoro-4-(methylaminocarboxamide) phenylam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com