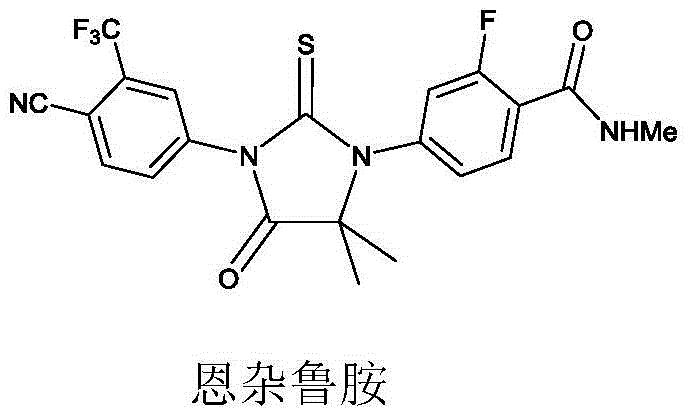
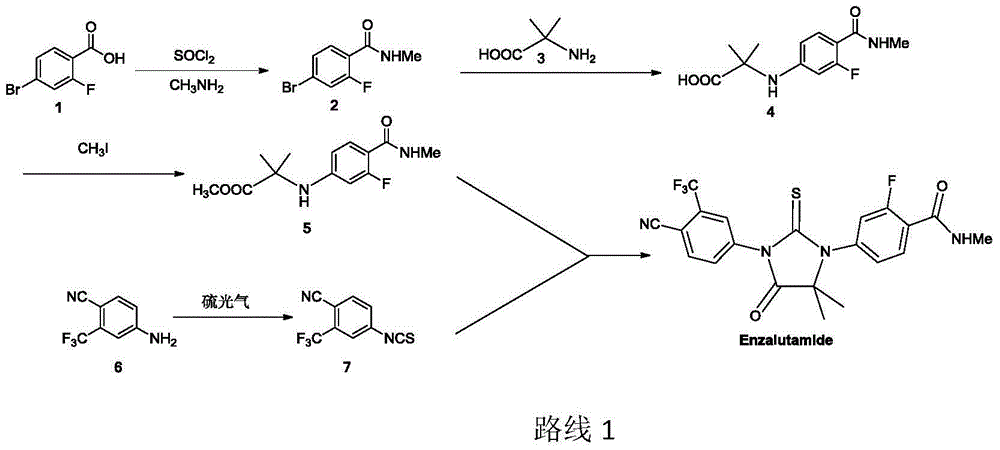
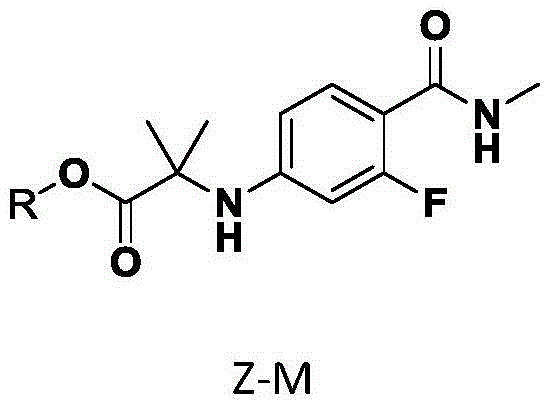
New compound for synthesizing Enzalutamide
A technology of enzalutamide and compound, which is applied in the field of synthesizing a new compound for treating prostate cancer drug enzalutamide and its preparation field, can solve problems such as unfavorable industrialized production, harm to laboratory personnel and environment, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1.2-(3-fluoro-4-(methylcarbamoyl) phenylamino) allyl 2-methylpropionate Z-M-1
[0025]
[0026] 2-(3-fluoro-4-(methylcarbamoyl)phenylamino)2-methylpropionic acid (10.0 g, 39.4 mmol), potassium carbonate (10.9 g, 78.8 mmol), allyl bromide ( 4.77g, 39.4mmol), DMF (100ml) was added into the reaction flask, under nitrogen protection, heated to 30°C, and stirred for 3 hours. Slowly add 800ml of ice water under stirring to precipitate a crystalline solid, continue to stir at room temperature for 2 hours, filter, rinse with 100ml of water, take out the filter cake and dry it in vacuum at 50°C to obtain 9.8g of white crystalline solid Z-M-1, HPLC purity 98.7%. The rate is 84.5%. ESI-MS(m / z): 295.14[M+H] + , 317.12[M+Na] + , 1 HNMR(DMSO-d6)δ: 1.480(s, 6H, 2CH 3 ), 2.728-2.740 (d, J=4.8, 3H, NCH 3 ), 4.555-4.575 (m, 2H, CH 2 ), 5.143-5.245 (m, 2H, CH 2 ), 5.769-5.866 (m, 1H, CH), 6.121-6.163 (dd, J 1 =2.0,J 2 =12.4, 1H, Ar-H), 6.293-6.320...
Embodiment 2
[0027] Example 2.2-(3-fluoro-4-(methylcarbamoyl) phenylamino) 2-methacrylic acid allyl ester Z-M-1 synthesis
[0028] 2-(3-Fluoro-4-(methylcarbamoyl)phenylamino)2-methylpropanoic acid (1.0 g, 3.94 mmol), potassium carbonate (1.09 g, 7.88 mmol), allyl bromide ( 0.95g, 7.88mmol), DMF (10ml) was added to the reaction flask, under nitrogen protection, heated to 30°C, and stirred for 3 hours. Slowly add 80ml of ice water under stirring to precipitate a crystalline solid, continue to stir at room temperature for 2 hours, filter, rinse with 10ml of water, take out the filter cake and dry it in vacuum at 50°C to obtain 0.96g of white crystalline solid Z-M-1, yield 83.0%.
Embodiment 3
[0029] Example 3.2-(3-fluoro-4-(methylcarbamoyl) phenylamino) 2-methacrylic acid allyl ester Z-M-1 synthesis
[0030]2-(3-Fluoro-4-(methylcarbamoyl)phenylamino)2-methylpropanoic acid (1.0 g, 3.94 mmol), potassium carbonate (1.09 g, 7.88 mmol), allyl bromide ( 1.43g, 11.82mmol), DMF (10ml) was added to the reaction flask, under nitrogen protection, heated to 30°C, and stirred for 3 hours. Slowly add 80ml of ice water under stirring to precipitate a crystalline solid, continue to stir at room temperature for 2 hours, filter, rinse with 10ml of water, take out the filter cake and dry it in vacuum at 50°C to obtain 0.91g of light yellow crystalline solid Z-M-1, yield 78.1% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com