Indazole hydrazide compound and application thereof
A technology of indazole hydrazide and compound, applied in the field of drug synthesis, can solve problems such as failure and weak anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0072] The present invention also provides a method for preparing the above-mentioned indazole hydrazide compound, comprising: reacting the compound represented by formula (II) with RCOR' to obtain the compound represented by formula (I).
[0073]
[0074] The compound represented by the formula (II) and RCOR' are preferably reacted in a solvent; the solvent is preferably an alcohol solvent, more preferably ethanol; the reaction is preferably a reflux reaction; the compound represented by the formula (II) The molar ratio to RCOR' is preferably 1:(1-1.2); in the examples provided by the present invention, it is specifically 1:1.1.
[0075] The compound represented by the formula (II) is preferably carried out according to the following steps: the compound represented by the formula (III) is reacted with hydrazine to obtain the compound represented by the formula (II).
[0076]
[0077] Among them, R″ is a C1-C5 alkyl group, more preferably a C1-C4 alkyl group, and still m...
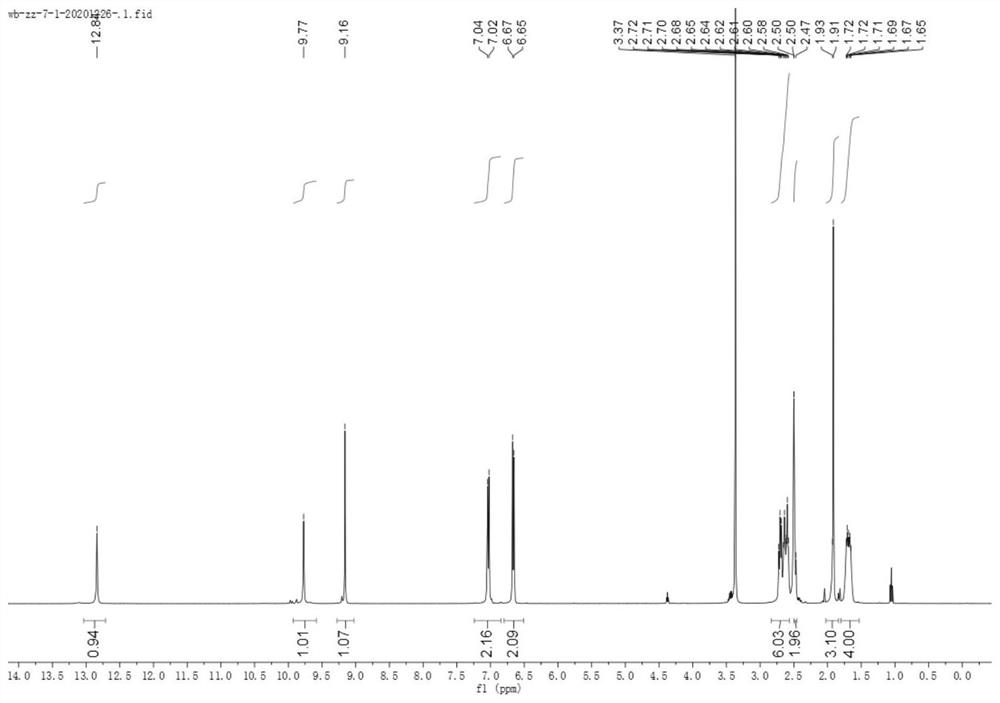
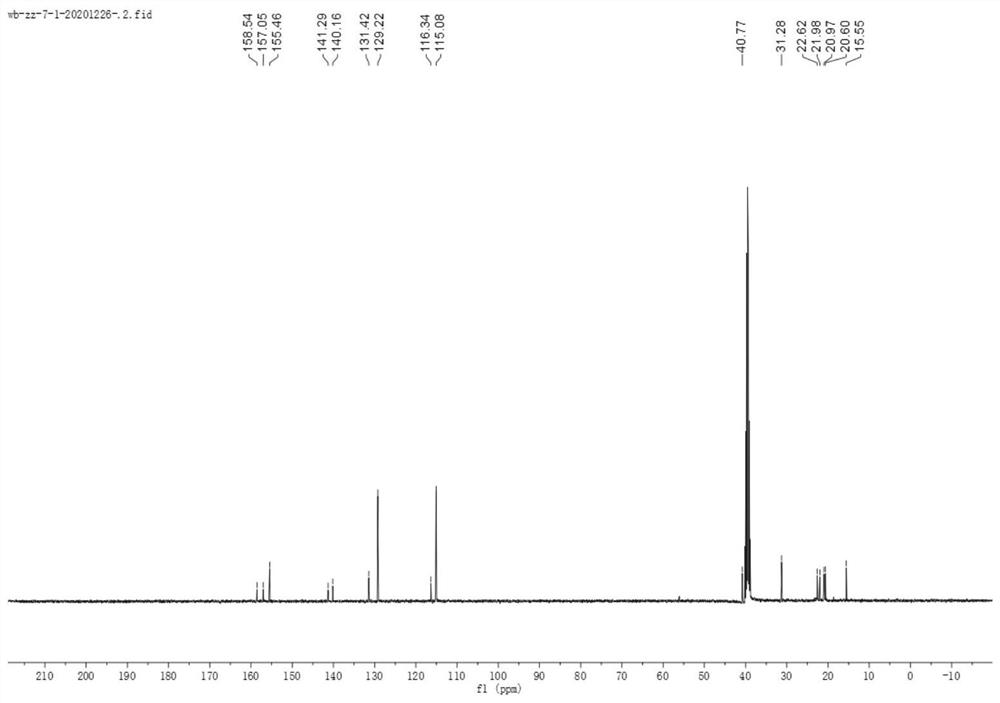
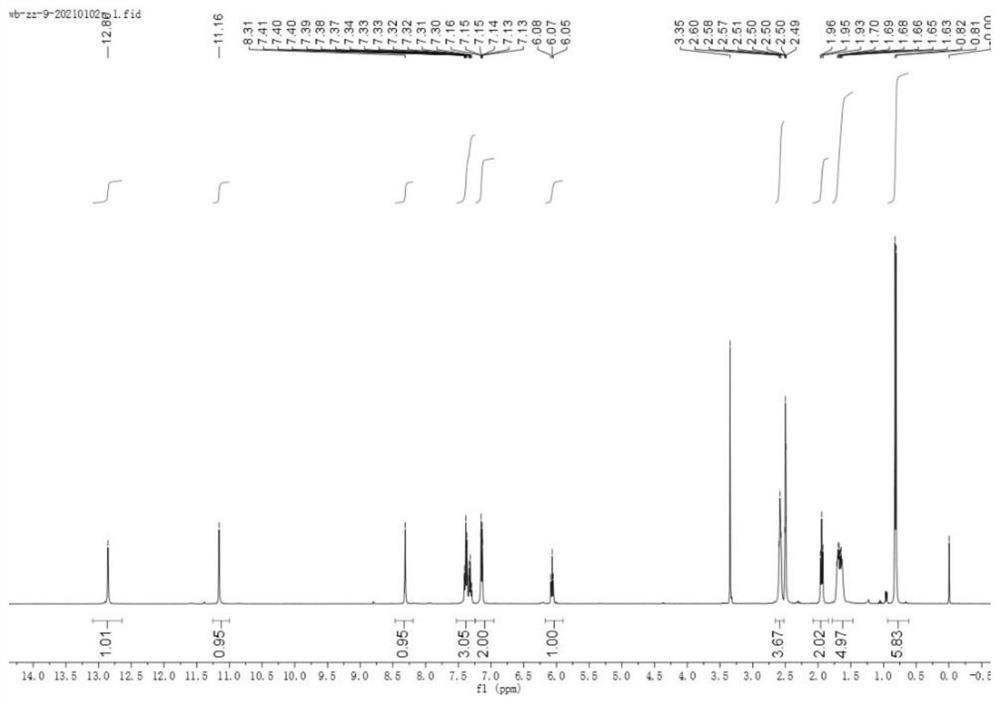
Embodiment 1
[0091] Compounds represented by formula (1), formula (2) to formula (10) were prepared according to the following steps:
[0092]
[0093] A sodium ethoxide solution was prepared by adding sodium (1.5 g, 65 mmol) to absolute ethanol (20 mL) at 0°C; then a mixture of cyclohexanone (4.41 g, 44 mmol) and diethyl oxalate (7.3 g, 50 mmol) was added slowly , and stirred the solution at room temperature for 12 h, and then decomposed the reaction mixture with 2N sulfuric acid solution, then extracted the mixture with ethyl acetate, dried and concentrated the organic solvent, and the obtained crude product was purified by n-hexane:ethyl acetate (12:1) column Further purification by chromatography gave product 2 as a yellow oil (5.87 g, 67%).
[0094] Hydrazine (448 mg, 14 mmol) was slowly added to a cooled suspension of product 2 (2.38 g, 12 mmol) in acetic acid (5 mL), the mixture was heated to reflux for 1 h, poured into ice water, washed with NaHCO 3 Neutralized and extracted wi...
Embodiment 3
[0135] Example 3: Detection of antitumor activity
[0136] 3.1 Experimental method
[0137] 3.1.1 Tumor cell recovery culture
[0138] Take out cryopreserved tumor cells (prostate cancer, ovarian cancer and other cells) from liquid nitrogen, dissolve quickly at 37°C, add 5ml of the corresponding medium containing FBS, centrifuge at 300g for 5 minutes, collect the precipitate, and resuspend in the corresponding medium Then, after thorough mixing, it was added to a 24-well cell culture dish, supplemented with medium to a total volume of 2 mL per well, incubated at 37°C to about 90% confluence, passaged, expanded or counted for plating.
[0139] 3.1.2 Tumor cell inoculation
[0140] After the tumor cells were cultured enough to be passaged, they were digested with trypsin and a single cell suspension was prepared. After counting the cells, adjust the cell concentration to 1.2×10 with the corresponding medium 4 cells / ml, add 90 μl of the mixture to a 96-well plate, incubate at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com