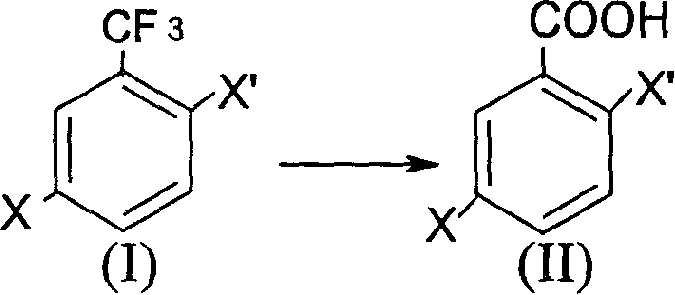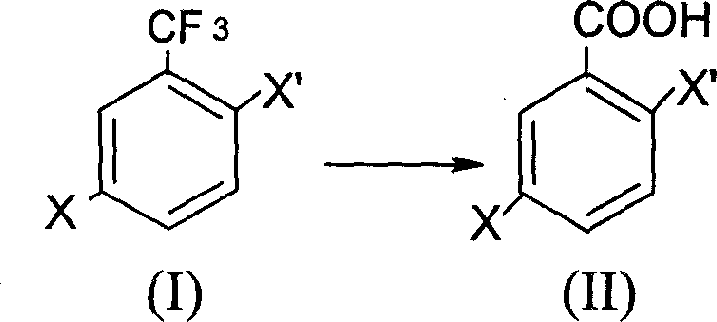Prepn process of 2,5-dihalogeno benzoic acid
A technology of dihalogenated benzoic acid and dihalogenated trifluorotoluene, which is applied in the field of preparation of dihalogenated benzoic acid, can solve the problems of complex process and high production cost, and achieve the effects of simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0014] Synthesis of 2-chloro-5-bromobenzoic acid
[0015] Add 41.5 g of 2-chloro-5-bromobenzotrifluoride to a four-necked flask equipped with a stirrer, a thermometer, a dropping funnel, and a reflux condenser, containing 20% SO 3 63g of oleum, heated to 58-65°C, continued to react at this temperature for 12 hours, cooled to below 50°C, added 200g of crushed ice, and filtered to obtain 35-40g of crude product. Recrystallized from toluene to obtain 28.5 g of the product with a purity of 99% and a yield of 75.6%.
[0016] m.p.: 154~156℃;
[0017] MS (m / z, M + ): 238;
[0018] IR (KBr, cm -1 ): 3300-2500, 3089, 2630, 2530, 1870, 1813, 1694, 1580, 1462, 845, 820, 781, 731, 657, 596.
[0019] Those skilled in the art can understand that the reaction temperature in the method of the present invention can be between 50-100°C. And the specific reaction time can be determined according to the actual product to be obtained. And can be selected according to the property of produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com