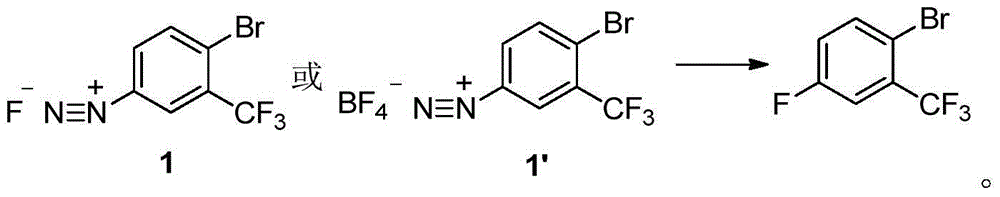
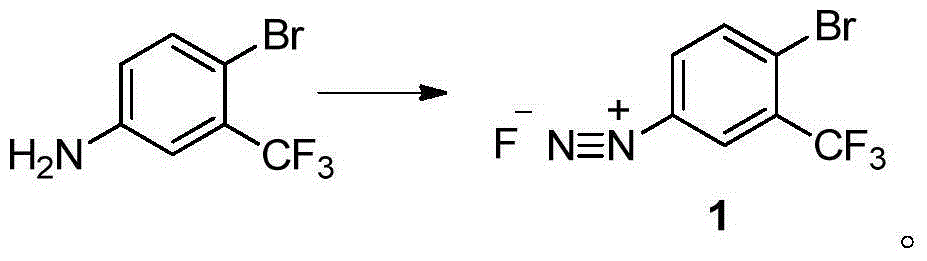
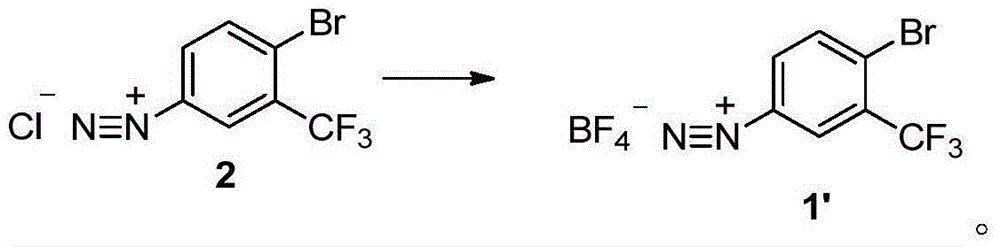
Method for preparing 2-bromine-5-trifluorotoluene chloride
A technology of fluorobenzotrifluoride and trifluoromethyl, which is applied in the field of preparation of 2-bromo-5-fluorobenzotrifluoride, can solve the problems of unsuitability for industrial production, expensive raw materials, harsh reaction conditions, etc. Effects of low isomer impurities, cheap raw materials, and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
[0093] Embodiment 1-7 carries out the reaction of amino protection to m-trifluoromethylaniline
[0094] Add 500g of m-trifluoromethylaniline into a four-neck flask, raise the temperature to 60°C, add 337g of acetic anhydride dropwise while stirring, and the dropwise addition time is 1 to 3 hours. More than 99%, the yield reaches more than 99%.
[0095] With reference to the above synthetic method, select different solvents and reaction temperatures, the conversion and yield obtained are shown in Table 1:
[0096] The reaction condition table of table 1 embodiment 2-8
[0097]
[0098] The calculation method of the conversion rate is: GC / HPLC determines the amount of unreacted raw materials, (feed amount - unreacted raw material) divided by the feed amount equals the conversion rate)
Embodiment 9-19
[0099] Embodiment 9-19 prepares the reaction of compound 4
[0100] Add 185 g of glacial acetic acid, 50 g of water, 100 g of sodium chlorate, and 5.3 g of iron powder to the reaction solution of Example 1 (the amount of compound 3 is about 3 mol), add 275 g of bromine dropwise, and the temperature is 65 ° C, and the dropwise addition is completed in 3 hours After 8 hours of heat preservation, 1300g of 5% sodium bisulfite was added dropwise to the reaction liquid, kept at 20°C to 30°C for 4h, filtered, the filter cake was washed with 1000g of water, and filtered to obtain the wet product 5-acetamido-2-bromotrifluoro Toluene, reaction conversion rate 99.5%, yield 98.6%.
[0101] Referring to the above synthesis method, different solvents, oxidants, and bromination reaction temperatures were selected to obtain Examples 10-19, and the specific data are shown in Table 2.
[0102] Table 2 Example 10 - 19 reaction condition table
[0103]
[0104]
[0105] The mass concentr...
Embodiment 20-26
[0106] Example 20-26 Removal of amino protecting group to prepare 3-trifluoromethyl-4-bromoaniline (reaction of removing amino protecting group)
[0107] Drop into 5-acetamido-2-bromobenzotrifluoride 1100g (HPLC purity ≥ 96%), mass percentage composition be 48% liquid caustic soda 200g (the described mass percentage composition refers to the hydrogenation in four-necked bottle) The mass of sodium accounts for the percentage of the total mass of sodium hydroxide aqueous solution), methanol 148.5g, temperature 90°C, heat preservation and stirring for 5 hours and then cooling to 65°C for stratification, the organic layer is washed with water and then stratified, the lower layer is 3-trifluoroform Base-4-bromoaniline, the reaction conversion rate is 99.5%, and the yield is 98.2%.
[0108] With reference to the above-mentioned synthetic method, different solvents, alkaline substances, and reaction temperatures were selected to obtain Examples 21-26, and the specific data are shown ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com