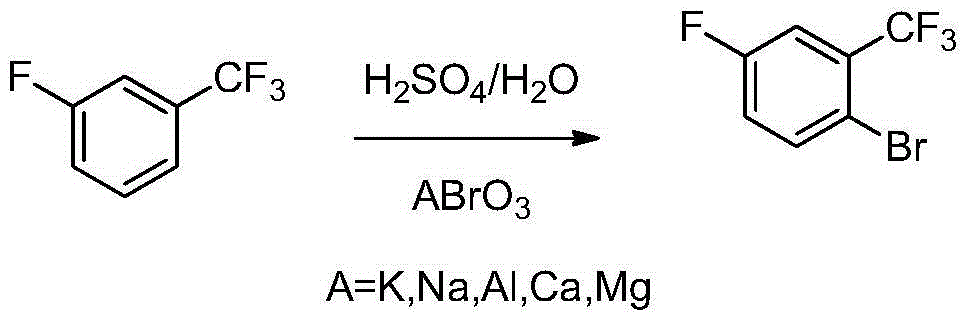Preparation method of 2-bromine-5-fluorine trifluorotoluene
A technology of fluorotrifluorotoluene and m-fluorotrifluorotoluene, which is applied in the field of organic synthesis, can solve the problems of difficulty in obtaining a brominating reagent, high processing costs, unfriendly environment, etc., and achieves low price, few reaction steps, and easy raw materials. effect of purchase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 6.00kg of 70% sulfuric acid into a 10L glass reactor, pass through frozen brine, control the temperature below 30°C, add 0.66kg of m-fluorobenzotrifluoride under stirring, heat the mixture to keep the temperature at 45-50°C, divide Add 0.80kg powdered potassium bromate in 10 batches for about 4 hours. After the addition, continue to keep warm and stir for 2 hours. Take samples for analysis until the reaction is complete, quench, extract with 3L dichloromethane, wash with sodium sulfite, remove the solvent after alkali washing, and rectify The 158-162°C / 760mmHg fraction was collected to obtain a product with a weight of 0.92kg, a purity of 99.2%, and a yield of 93.9%.
Embodiment 2
[0025] Add 6.00kg of 70% sulfuric acid into a 10L glass reactor, pass through frozen brine, control the temperature below 30°C, add 0.66kg of m-fluorobenzotrifluoride under stirring, heat the mixture to keep the temperature at 45-50°C, divide Add 1.00kg powdered potassium bromate in 10 batches for about 4 hours. After the addition, continue to keep warm and stir for 2 hours. Take samples for analysis until the reaction is complete, quench, extract with 3L dichloromethane, wash with sodium sulfite, remove the solvent after alkali washing, and rectify The 158-162°C / 760mmHg fraction was collected to obtain a product with a weight of 0.91kg, a purity of 99.0%, and a yield of 92.7%.
Embodiment 3
[0027] Add 6.00kg of 70% sulfuric acid into a 10L glass reactor, pass through frozen brine (water bath, the same below), control the temperature below 30°C, add 0.66kg of m-fluorobenzotrifluoride under stirring, and heat the mixture to keep the temperature at 30~35℃, add 0.80kg powdered potassium bromate in 10 batches, about 4 hours, continue to keep warm and stir for 2 hours after adding, take samples and analyze until the reaction is completed, quench, extract with 3L dichloromethane, wash with sodium sulfite, wash with alkali After precipitating, rectification collects 158~162 ℃ / 760mmHg distillate, obtains product weight 0.90kg, purity 99.1%, yield 91.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com