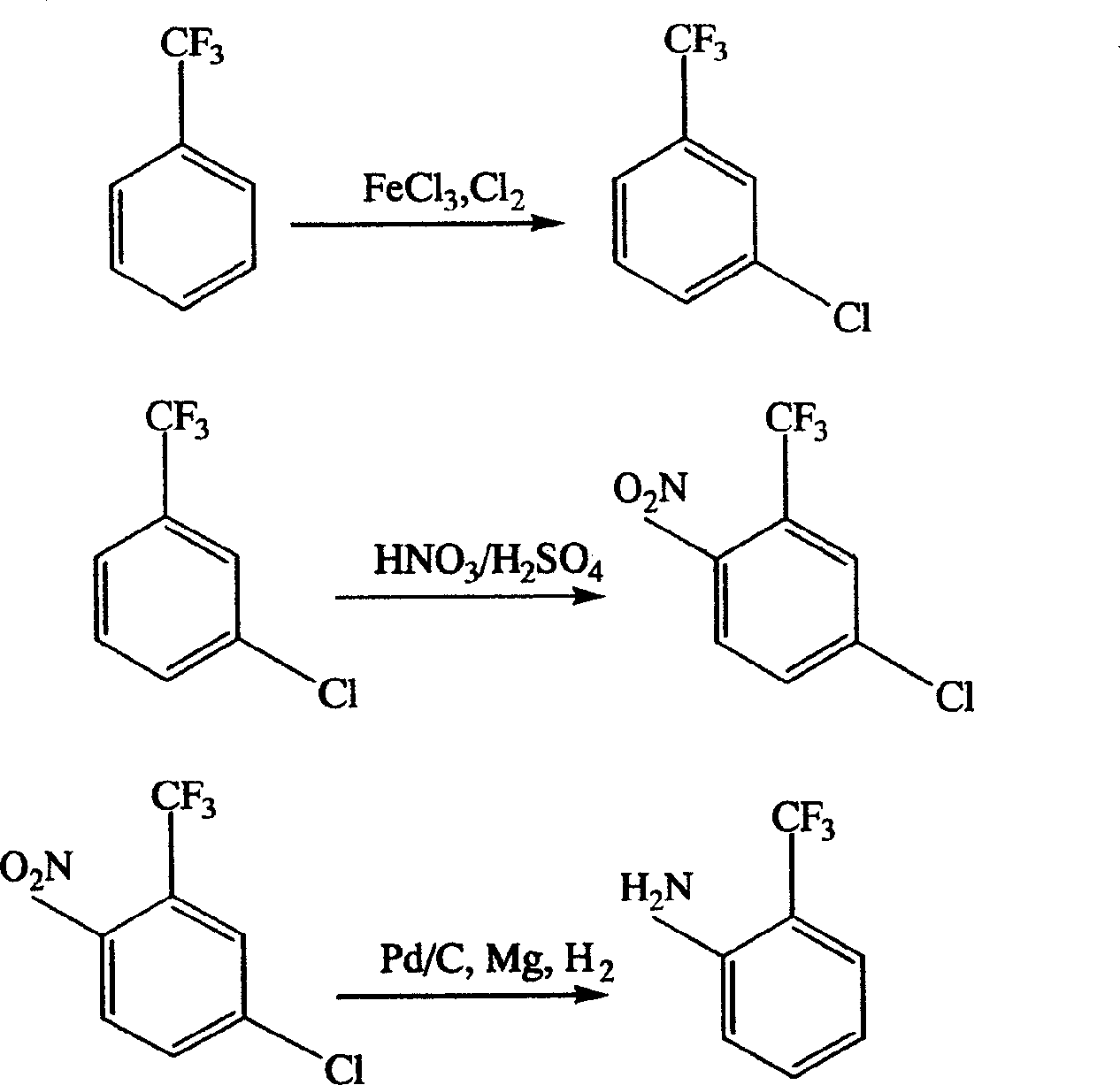
Process for preparing o-trifluoromethyl aniline
A technology of trifluoromethylaniline and trifluoromethylbenzene, which is applied in the field of hydrogenolysis dechlorination technology and the preparation of o-trifluoromethylaniline, can solve the problems of unavailable raw materials, long synthetic route, poor process technology, etc., and achieve the best results Good, short synthetic route, advanced technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
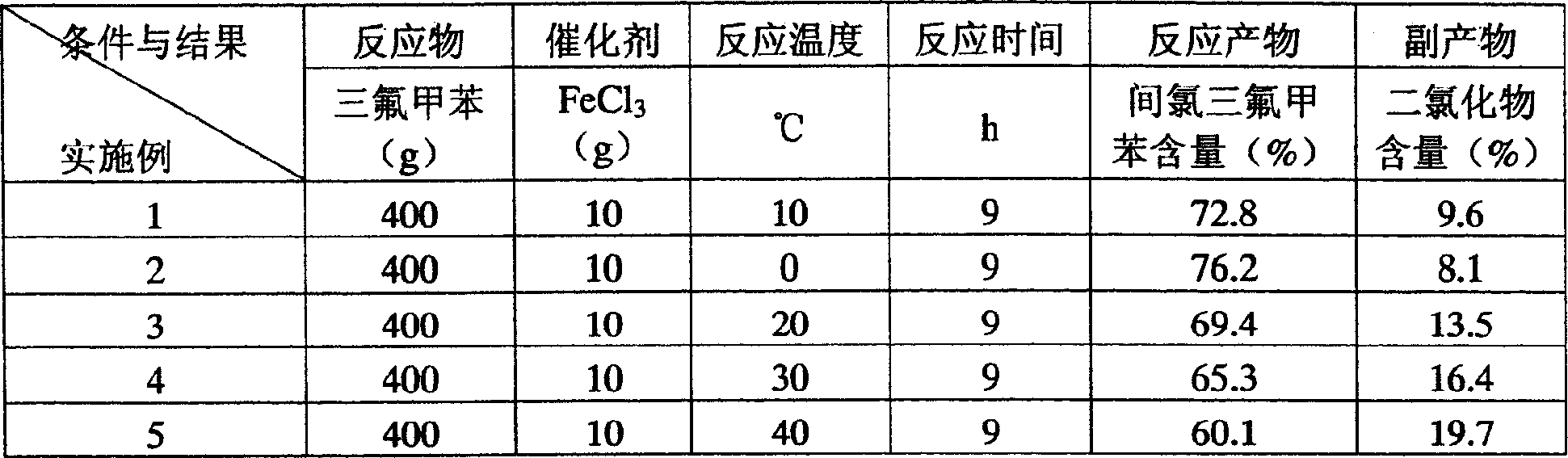
[0018] Example 1: In a 1000 ml flask, put 400 g of trifluorotoluene and 10 g of ferric chloride into the flask, keep the reaction temperature at 10-15° C., feed chlorine gas for 9 hours under stirring, and after the reaction is complete, use compressed air to drive out the reaction flask. Residual chlorine and hydrogen chloride gas, filter to remove the catalyst, the filtrate is analyzed by gas chromatography, the content of each component is: 4.8% benzotrifluoride, 72.8% m-chlorobenzotrifluoride, 4.2% o-chlorobenzotrifluoride, 6.2% p-chloro Trifluorotoluene, 12% as polychlorides. The reaction liquid is rectified, and the 120-130°C / 0.04Mpa (vacuum degree) fraction is collected, which is a mixture of ortho, meta and para isomers with m-chlorobenzotrifluoride as the main content.
Embodiment 2-5
[0019] Embodiment 2-5 is the same as embodiment 1.
[0020] Table 1 Quantitative data table of the influence of main reaction conditions on the reaction in the ring chlorination reaction
[0021]
[0022] Nitration of a mixture of m-chlorobenzotrifluoride and its isomers:
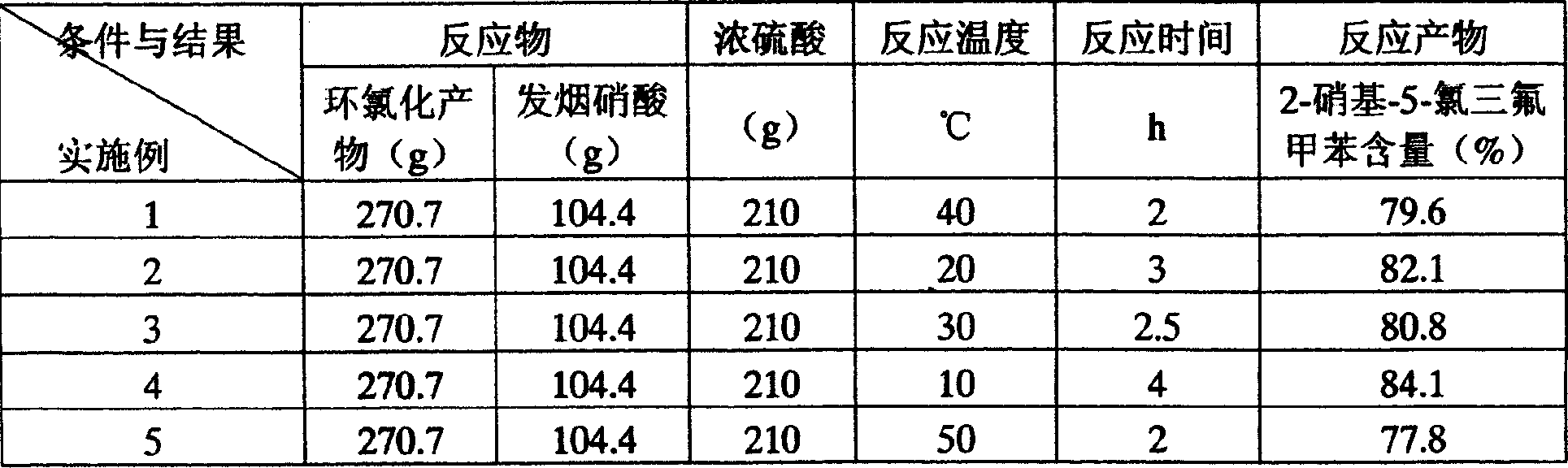
[0023] Embodiment 1: In a 1000ml flask, drop into 210g of concentrated sulfuric acid, under cold water cooling, dropwise add 104.4g of fuming nitric acid, and keep the reaction temperature at 10-15°C, add dropwise 270.7g of m-chlorotrifluorotoluene and its iso The mixture of conformers was added dropwise for about 1 hour. After the dropwise addition, react at 45-50°C for 2 hours. After the reaction, the waste acid in the lower layer is separated, and the nitrated product in the upper layer is washed with water and neutralized. The main content of the mixture of various isomers, the content is 79.6%.
[0024] Embodiment 2-5 is the same as embodiment 1.
[0025] Table 2 Quantitative data table ...
Embodiment 2-9
[0029] Embodiment 2-9 is the same as embodiment 1.
[0030] Table 3 Quantitative data table of the influence of main reaction conditions on the reaction in catalytic hydrogenation-hydrogenolysis dechlorination reaction
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com