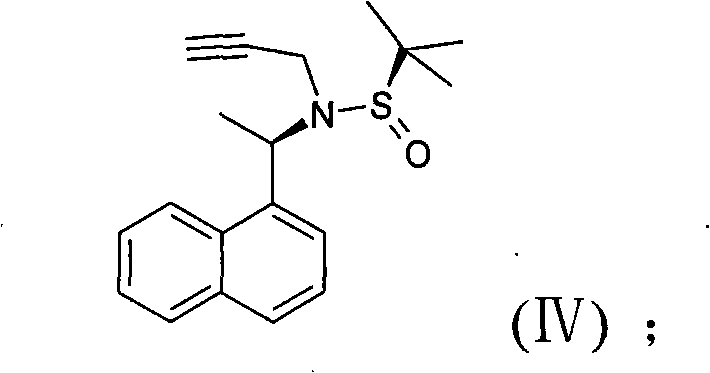
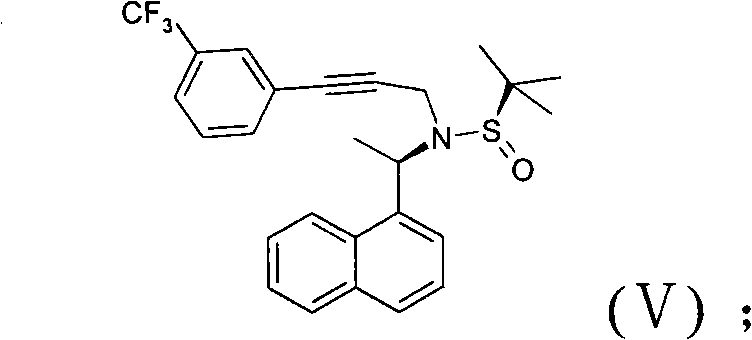
Novel preparation method of cinacalcet hydrochloride
A technology for cinacalcet hydrochloride and a compound, which is applied to the new preparation field of cinacalcet hydrochloride, can solve problems such as unfavorable industrial production, need to split naphthalene ethylamine, serious side reactions, etc., and achieves low cost and little environmental pollution. , the effect of convenient source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0046] The present invention will be further described below in conjunction with a specific embodiment, but the present invention is not limited to the following embodiment.
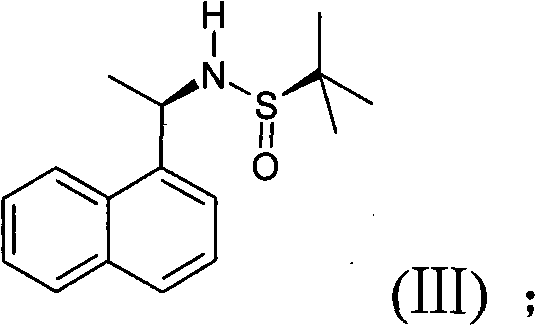
[0047] The first step: preparation of compound (III)
[0048] Add 1-naphthylethanone (1.7g) into a 100mL round bottom flask, dissolve it with tetrahydrofuran (20mL), then add (R)-tert-butylsulfinamide (2.2g), and heat the reaction mixture to 70°C , and then add 2.8g of isopropyl titanate to the flask, after about 10min, keep the reaction for 22h. The completion of the reaction was monitored by TLC, and then the temperature was lowered to about 0°C. Sodium borohydride (0.5g) was dissolved in tetrahydrofuran (3mL), cooled to 0°C, and slowly added to the above reaction mixture for about 10min. After the addition, heated to room temperature and kept stirring for 5h. After the reaction was monitored by TLC, it was cooled to 0°C. Stir for another 5min, filter the resulting suspension, wash the filter cake w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com