Preparation method of 3,5-dihalobenzotrifluoride and 3'-chloro-5'-(trifluoromethyl)phenyltrifluoroethanone
A technology of dihalotrifluorotoluene and phenyl trifluoroethyl ketone is applied in the field of chemical pharmacy, and can solve the problems of affecting the economic effect of enterprises, increasing the production cost of enterprises, complicated production process and the like, and achieving low price, low production cost, The effect of simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
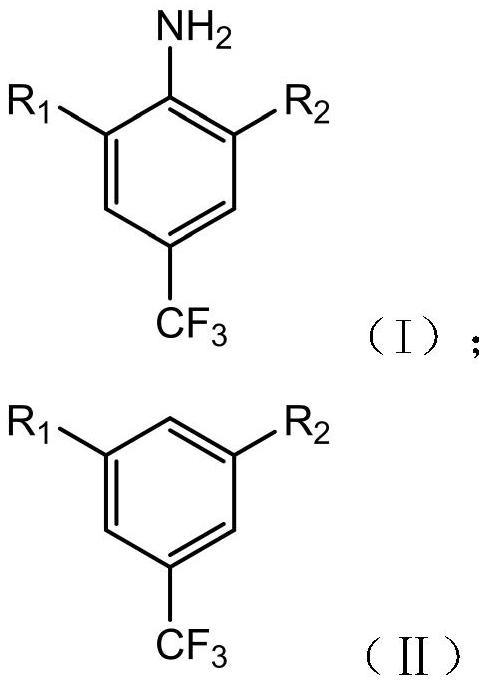
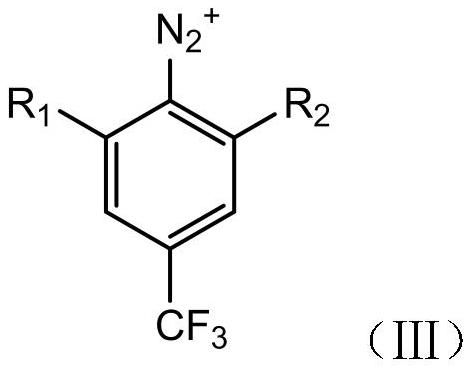
[0049] A method for preparing 3,5-dihalogenobenzotrifluoride, which uses 3,5-dichlorobenzotrifluoride (compound I) as a raw material to prepare compound II through the following steps.
[0050] S1. Dissolve 0.2mol of compound 1 in 200mL of solvent I, and cool down to 0°C to obtain reaction solution I;
[0051] S2. 84g of sulfuric acid with a mass fraction of 92.5% was added dropwise to the reaction solution I, and the dropwise addition process was completed within 30 minutes. After the dropwise addition, the reaction was continued for 1 hour to obtain the reaction solution II;
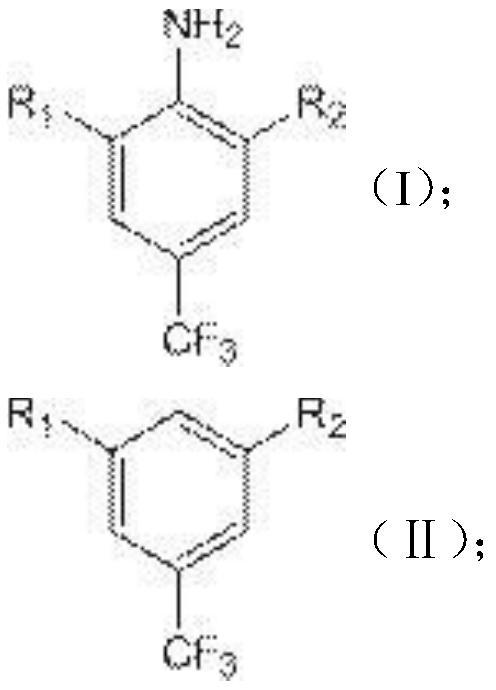
[0052] S3. Add a nitrous acid reagent solution containing 0.44 mol of nitrous acid reagent to the reaction solution II, keep the temperature of the system below 0° C., and keep the reaction for 2 hours to obtain the reaction solution III;
[0053] S4. The temperature of the reaction solution III was raised to 20°C, and 87 g of 50% hypophosphorous acid was evenly added within 60 minutes, and 0.5 g of cu...
Embodiment 2~6
[0060] A preparation method of 3,5-dihalogenotrifluorotoluene, the difference from Example 1 is that R 1 and R 2 different. R in Examples 2-6 1 and R 2 As shown in Table 1.
[0061]
Embodiment 7~8
[0063] A method for preparing 3,5-dihalogenotrifluorotoluene, the difference from Example 1 is that in step S1, ethanol and isopropanol are selected as solvent I respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com