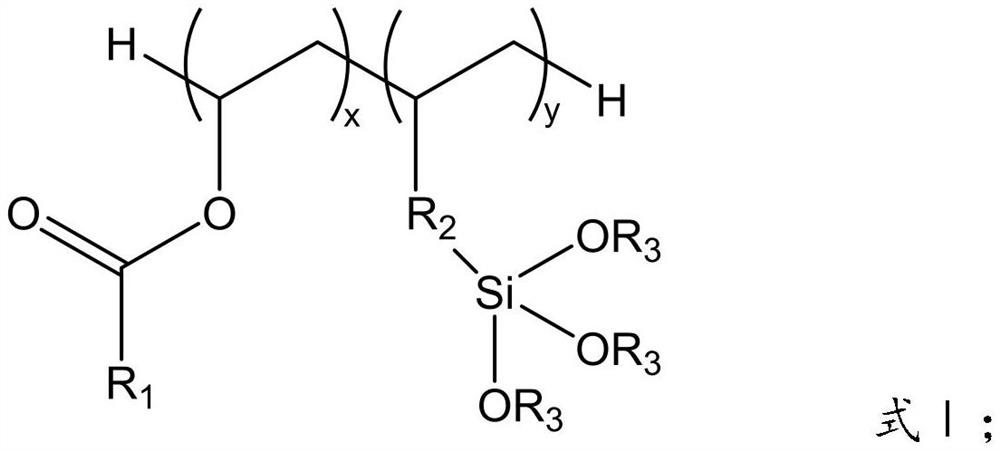
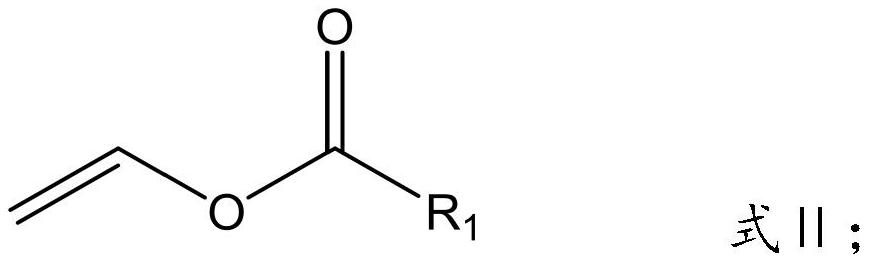
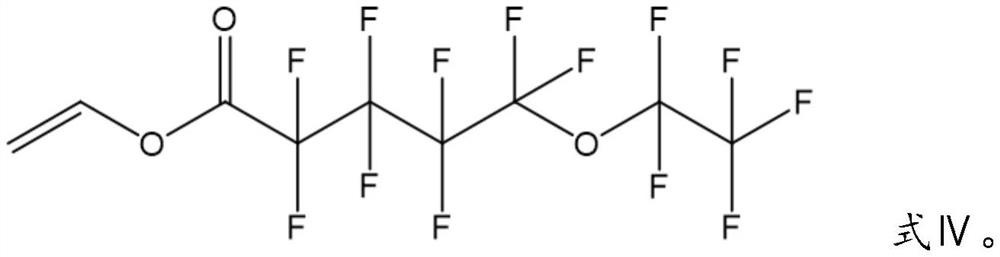
Patents
Literature
65 results about "Fluoroethyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fluoroethyl is an organofluorine functional group in chemistry.
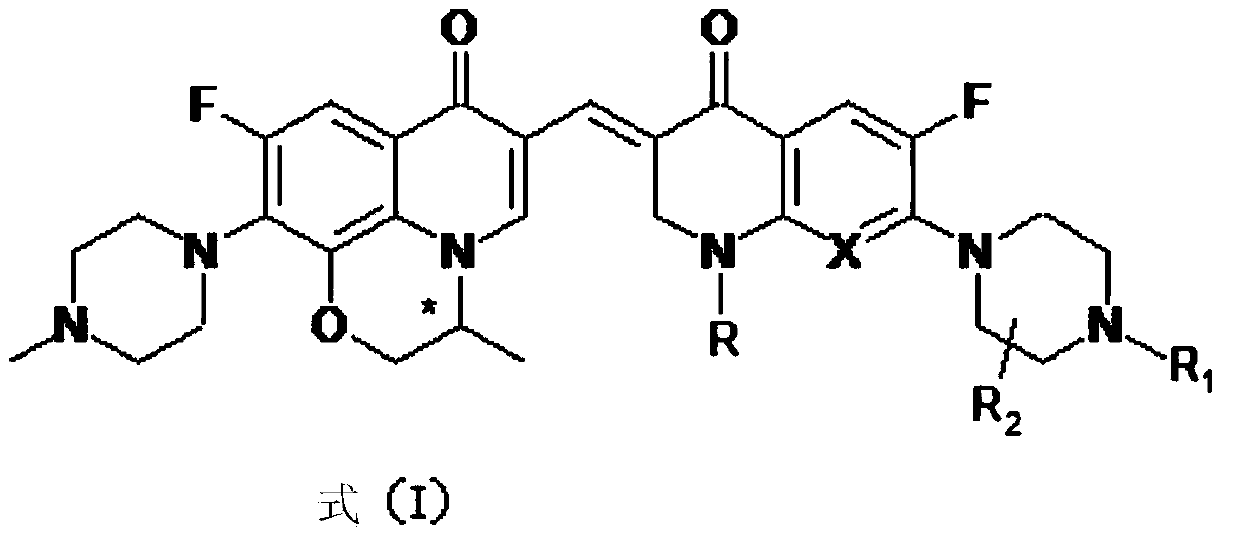
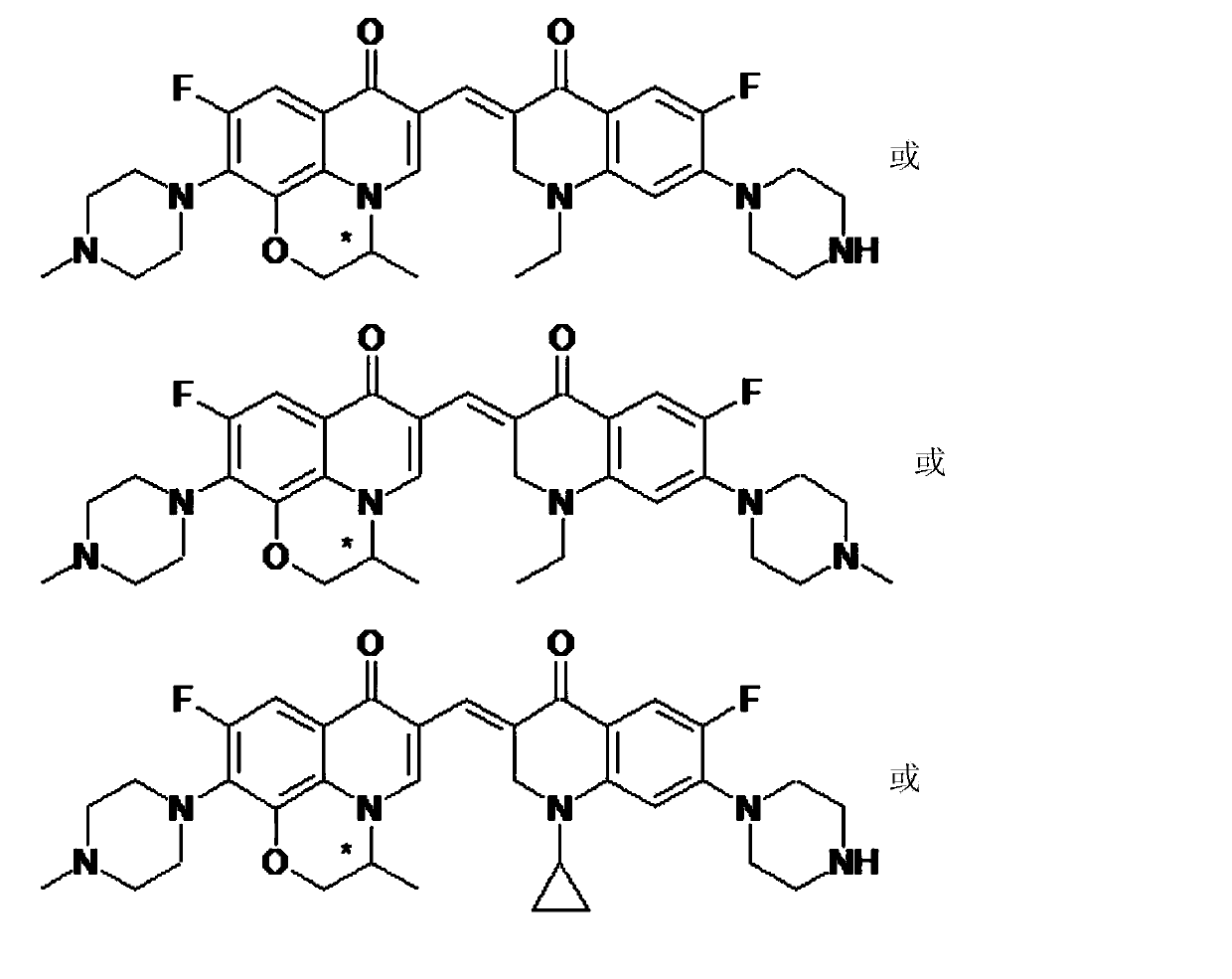
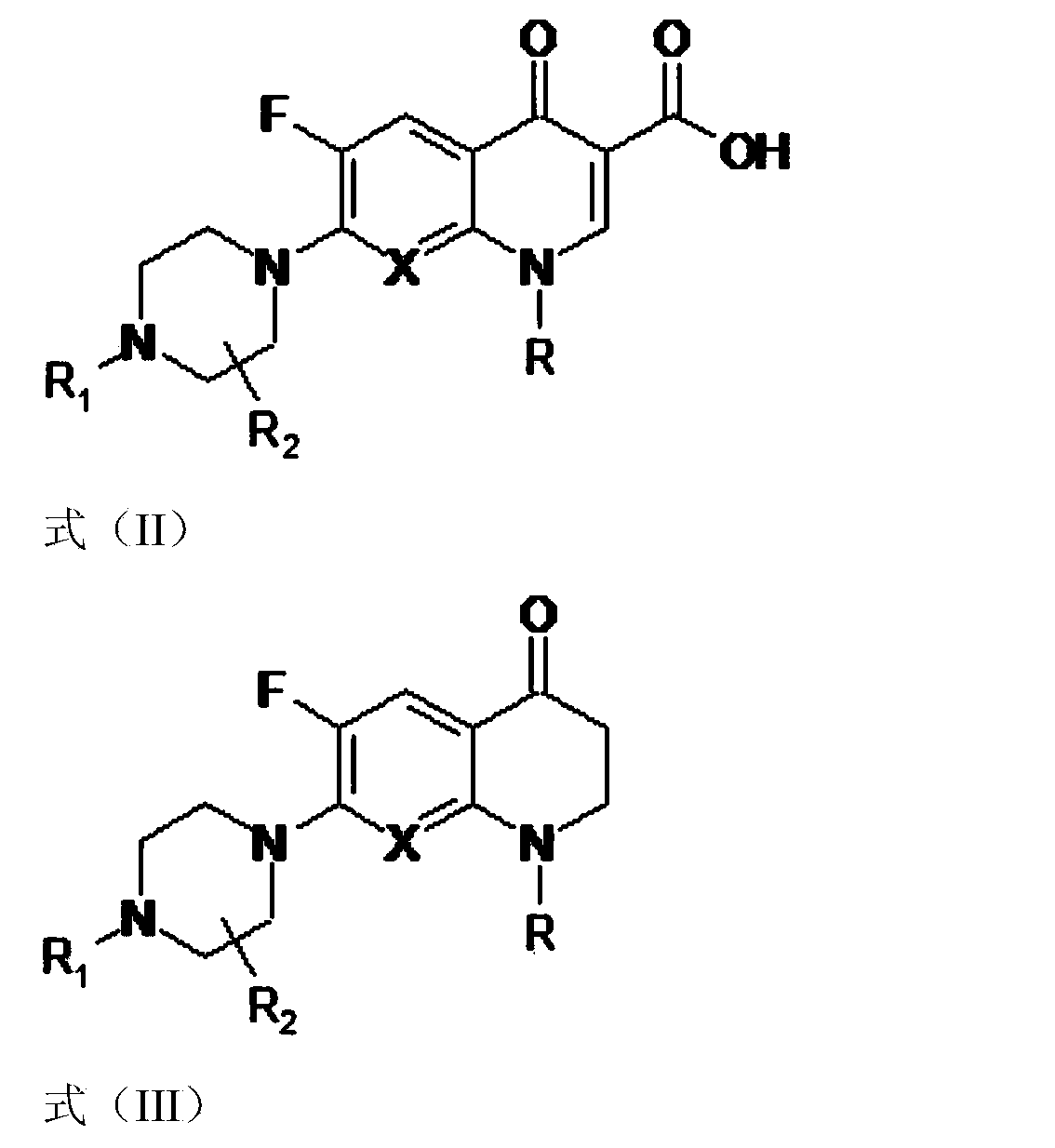
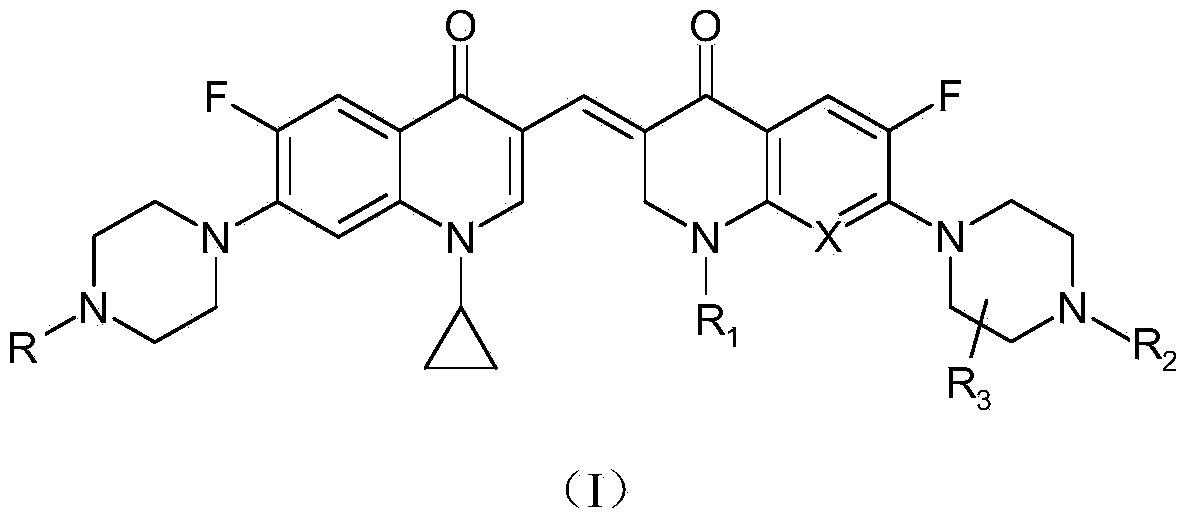
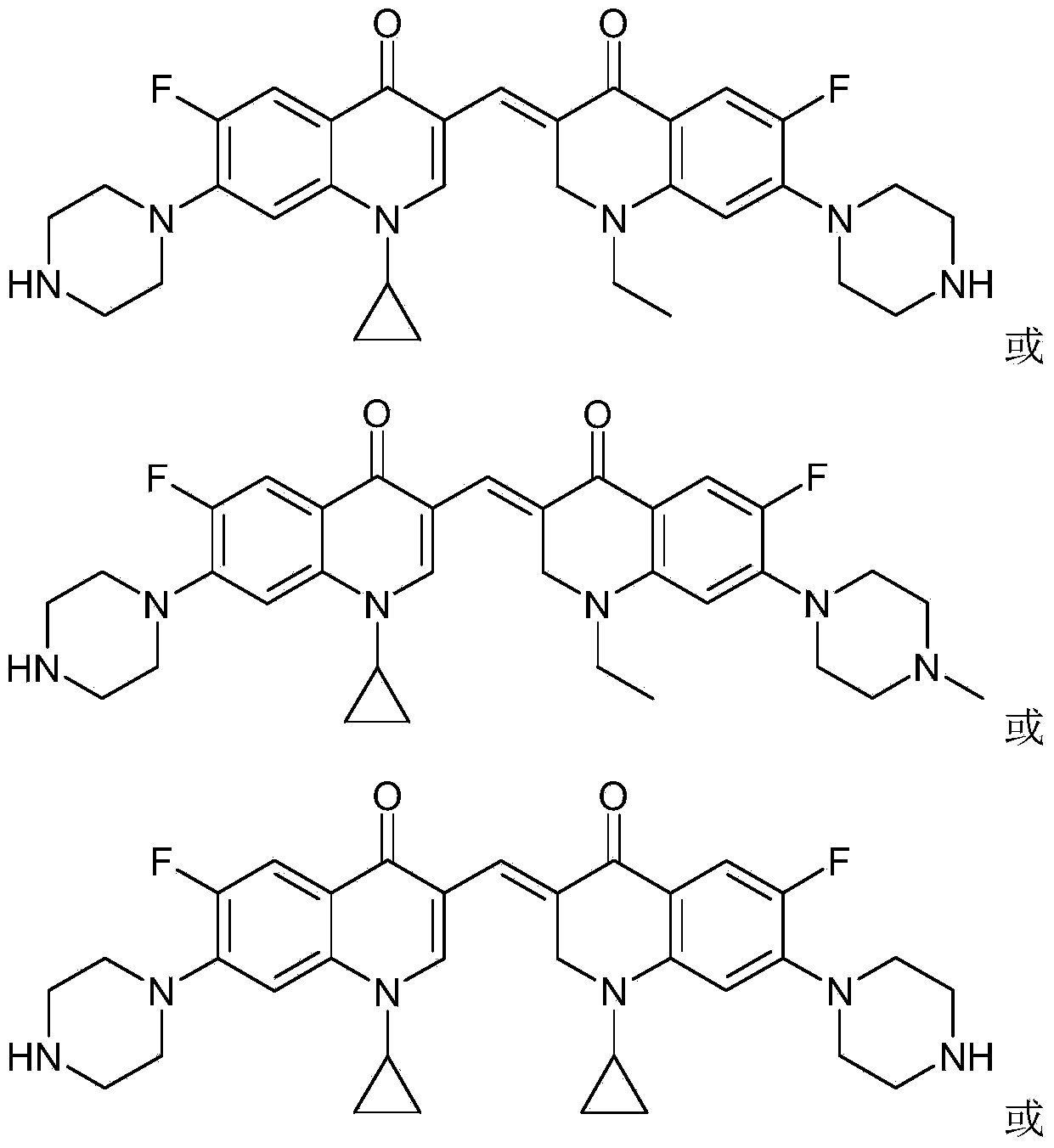
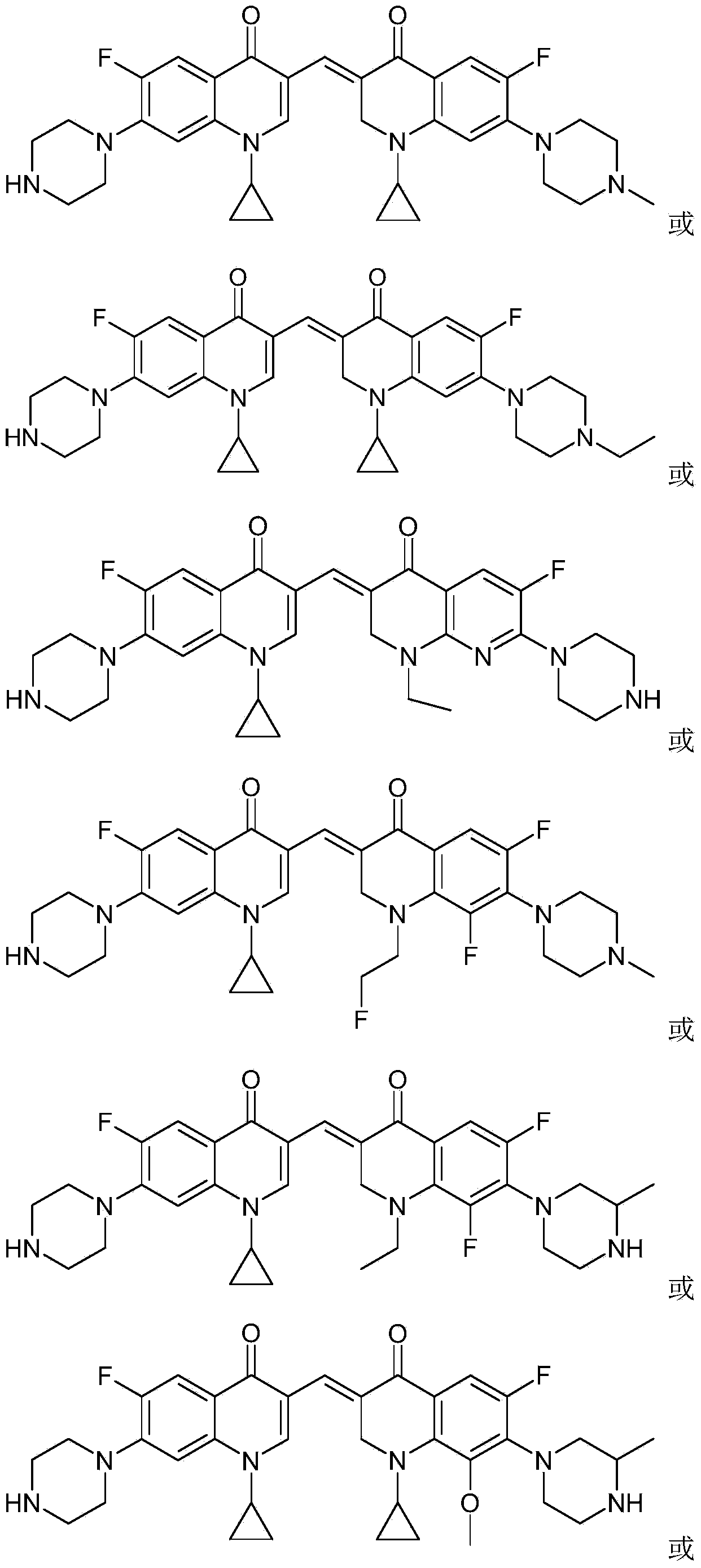
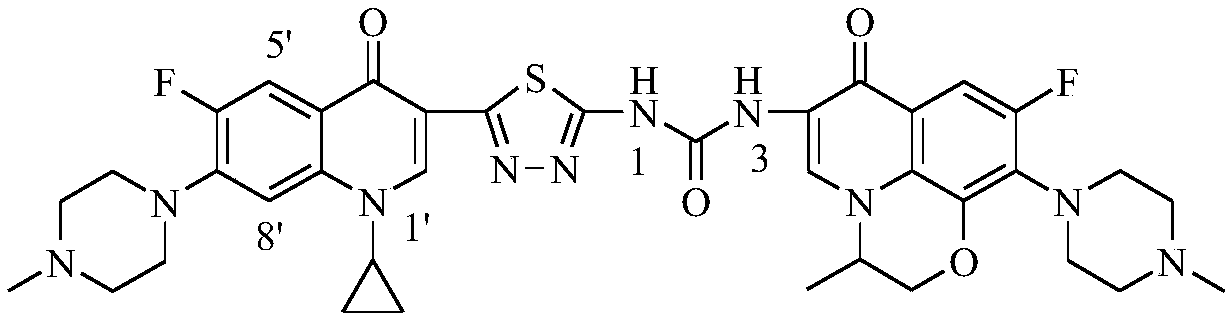
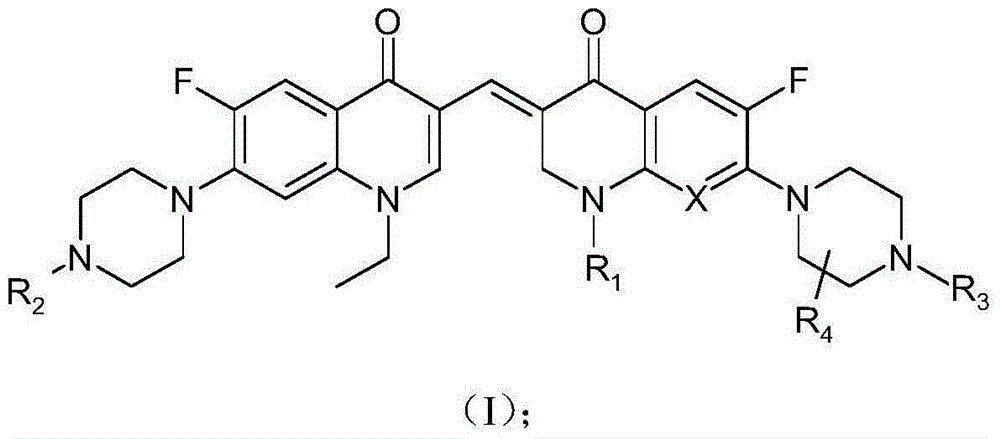
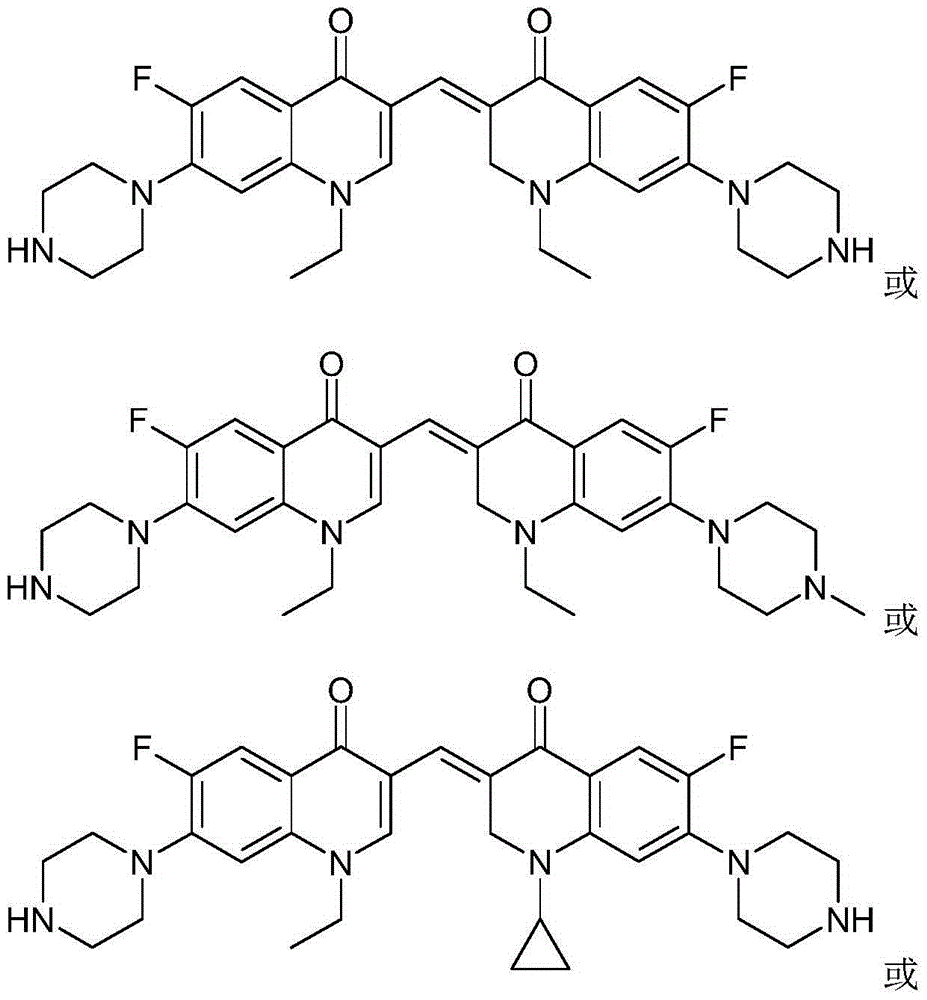
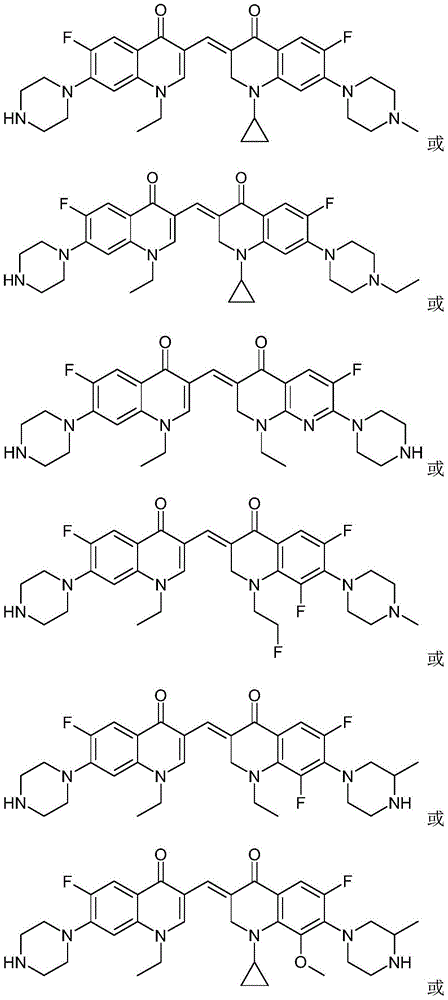
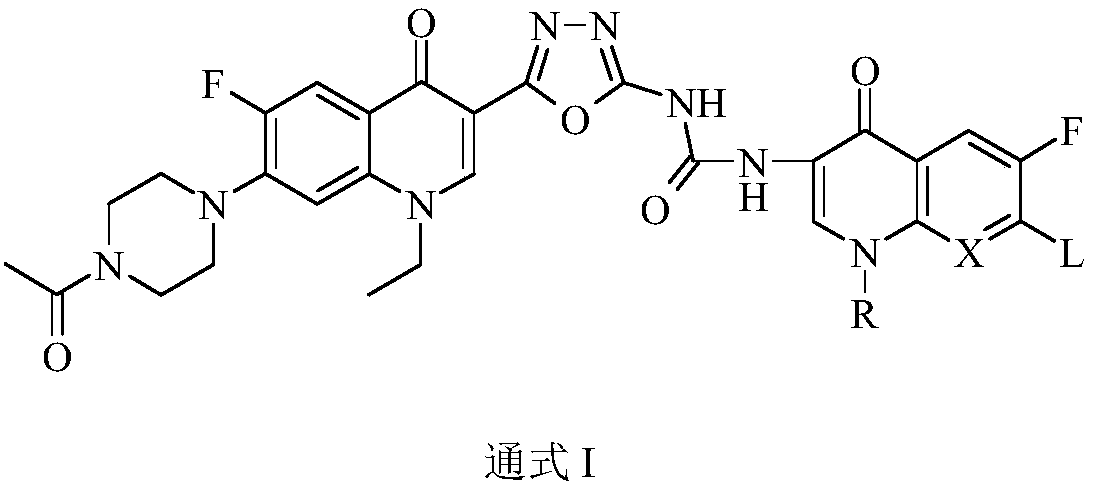
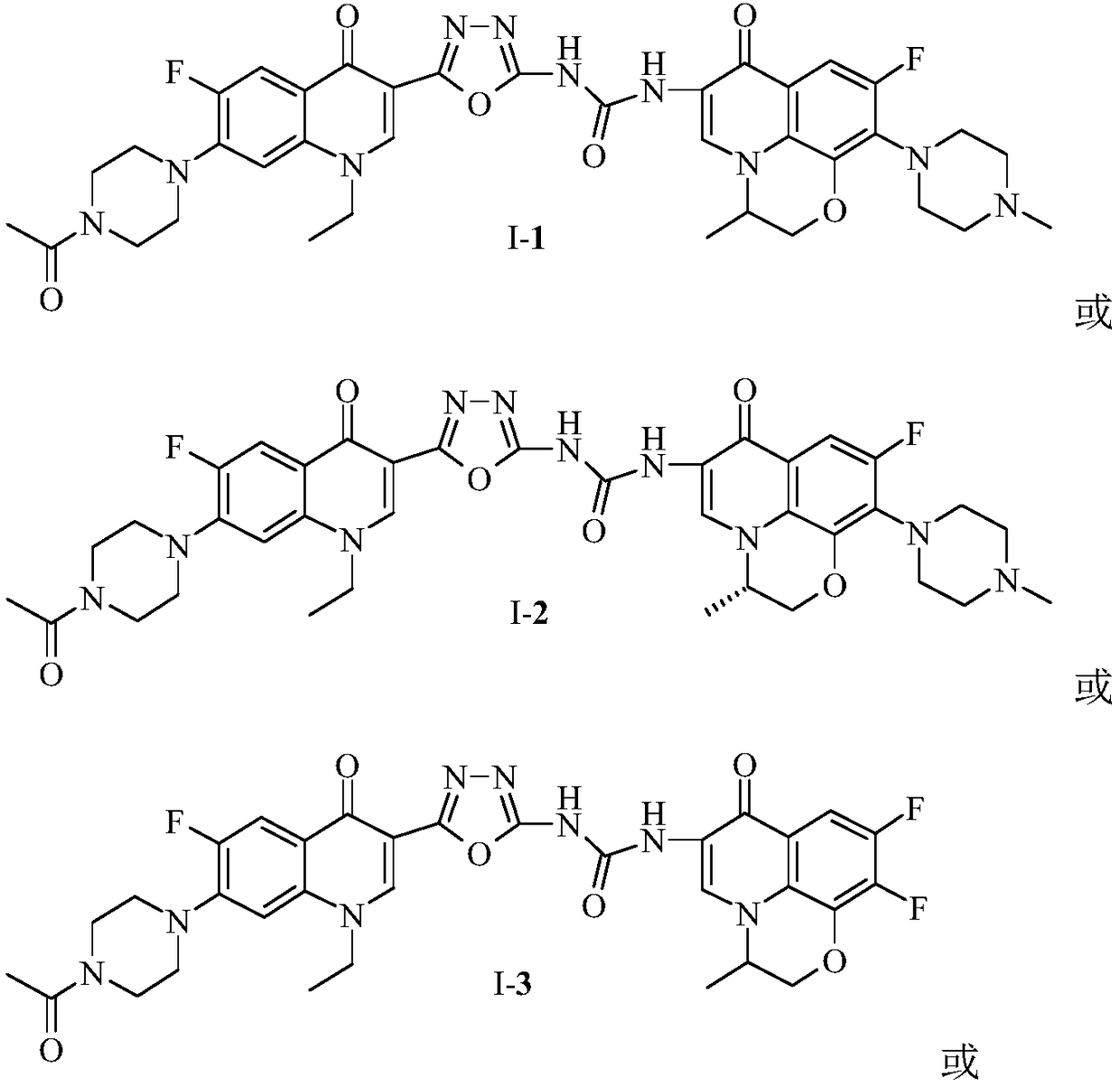
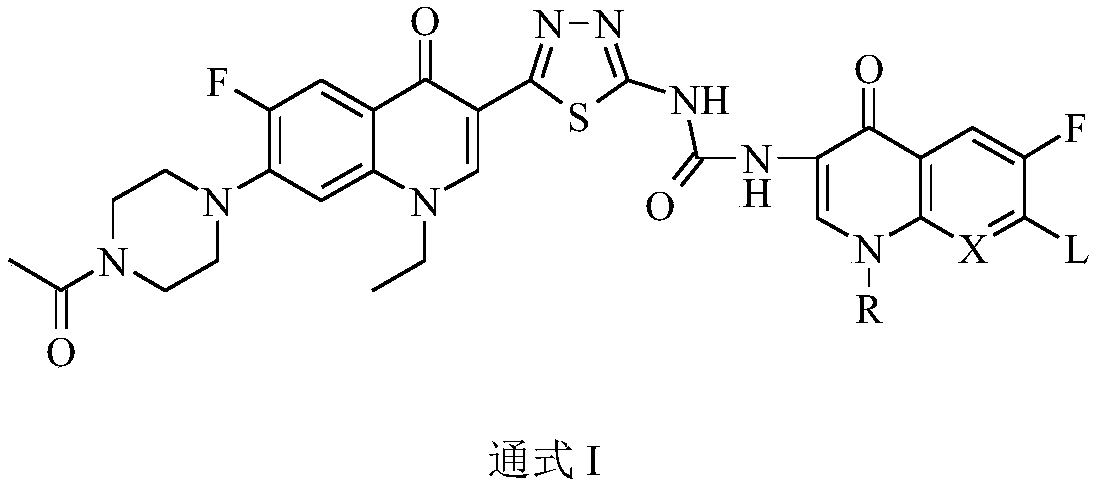
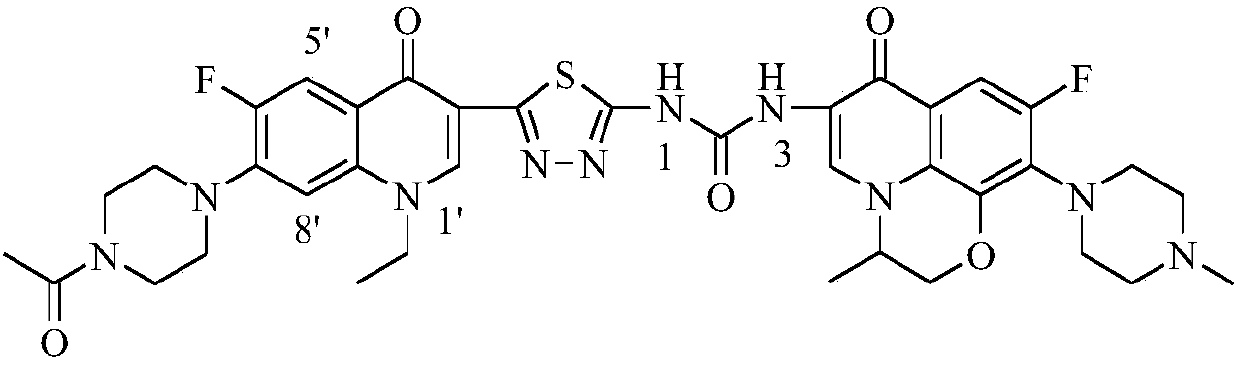
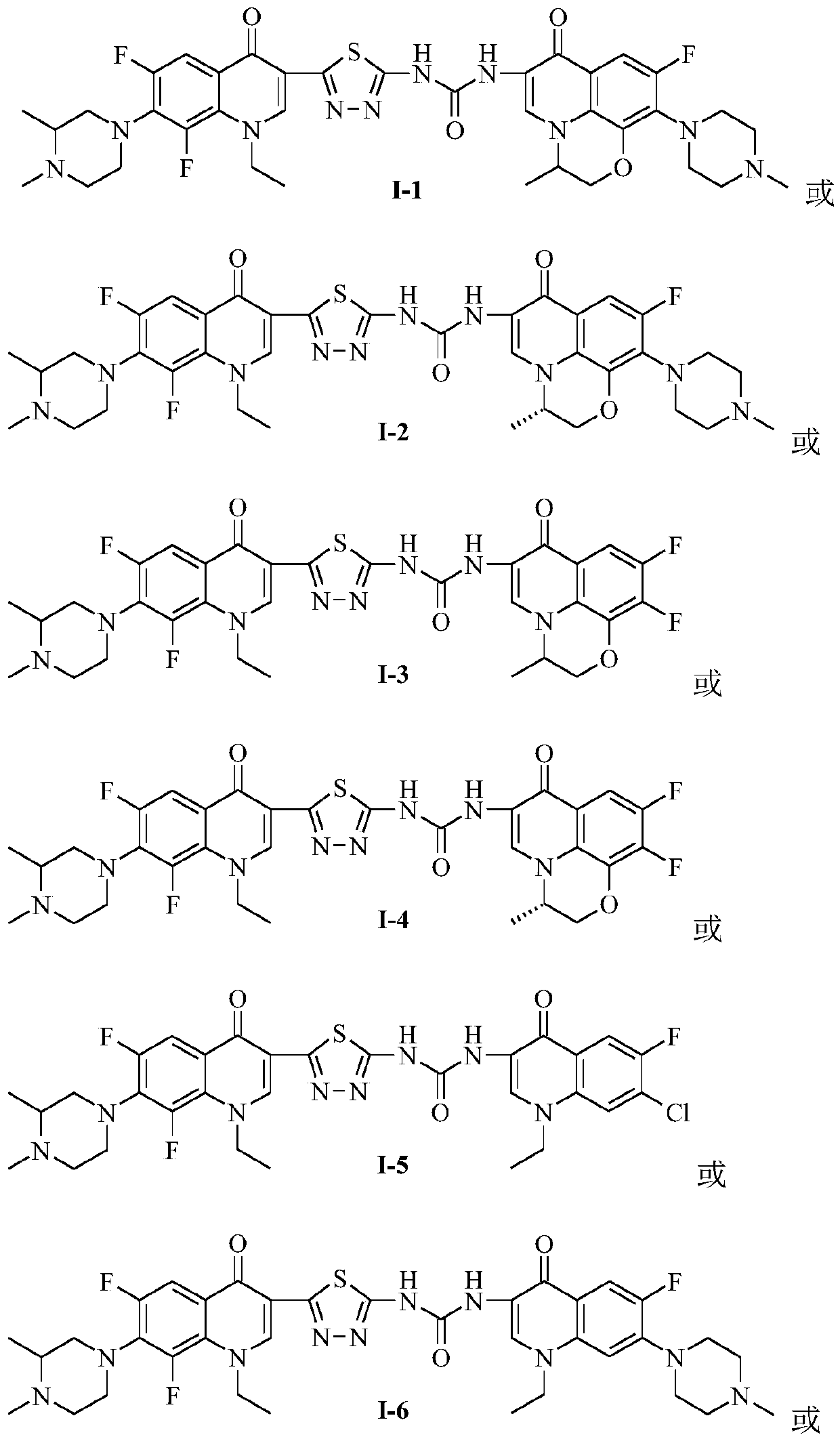
3,3'-methene-difluoroquinolone derivative of chiral oxazine quinoline ring as well as preparation method and application of 3,3'-methene-difluoroquinolone derivative
InactiveCN104557973AStrong complementarityTo achieve the effect of increasing efficiency and reducing toxicityOrganic active ingredientsOrganic chemistrySide effectPharmacophore
The invention discloses a 3,3'-methene-difluoroquinolone derivative of a chiral oxazine quinoline ring as well as a preparation method and application of the 3,3'-methene-difluoroquinolone derivative. The 3,3'-methene-difluoroquinolone derivative has a chemical general structural formula I shown in the specification, wherein R represents cyclopropyl or ethyl or fluoroethyl; R1 represents a hydrogen atom or methyl or ethyl; R2 represents a hydrogen atom or methyl; X represents a nitrogen atom or a hydrocarbon (CH) group or a fluoro-substituted carbon atom (F-C) or a methoxyl-substituted carbon atom (CH3O-C). The 3,3'-methene-difluoroquinolone derivative of the chiral oxazine quinoline ring, disclosed by the invention, can be used for realizing the superposition of a difluoroquinolone framework and alpha, beta-unsaturated ketone pharmacophores, so that the antitumor activity of a new compound is improved, the toxic and side effects on normal cells can be reduced, and the 3,3'-methene-difluoroquinolone derivative can be used as an antitumor active substance for developing an antitumor drug of a totally new structure.
Owner:HENAN UNIVERSITY
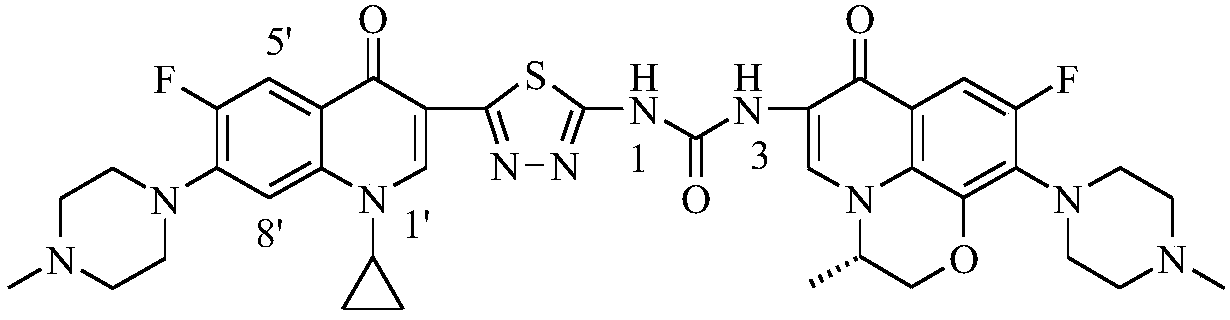
3,3'-methylene-bisfluoroquinolone derivative containing cyclopropylquinoline ring as well as preparation method and application of 3,3'-methylene-bisfluoroquinolone derivative
InactiveCN104370812ASmall toxicityAchieve overlayOrganic chemistryAntineoplastic agentsFluoroethylNormal cell
The invention discloses a 3,3'-methylene-bisfluoroquinolone derivative containing a cyclopropylquinoline ring as well as a preparation method and application of the 3,3'-methylene-bisfluoroquinolone derivative. The 3,3'-methylene-bisfluoroquinolone derivative containing the cyclopropylquinoline ring is a compound having a structural general formula as shown in the specification, wherein R is H, methyl or ethyl; R1 is ethyl, cyclopropyl or fluoroethyl; R2 is H, methyl or ethyl; R3 is H or methyl; and X is CH, N, F-C or CH3O-C. According to the 3,3'-methylene-bisfluoroquinolone derivative containing the cyclopropylquinoline ring, based on the combination principle of pharmacophores, the superposition of bisfluoroquinolone pharmacophore and alpha, beta-unsaturated ketone is achieved, and a 'fluoroquinolone chalcone' derivative is designed and synthesized; by the structural complementation, the anti-tumor activity is increased, the toxic or side effect on normal cells is decreased so as to achieve synergistic and toxicity-attenuation effects and the 3,3'-methylene-bisfluoroquinolone derivative can be used as an anti-tumor active substance to develop an anti-tumor drug having a new structure.
Owner:HENAN UNIVERSITY
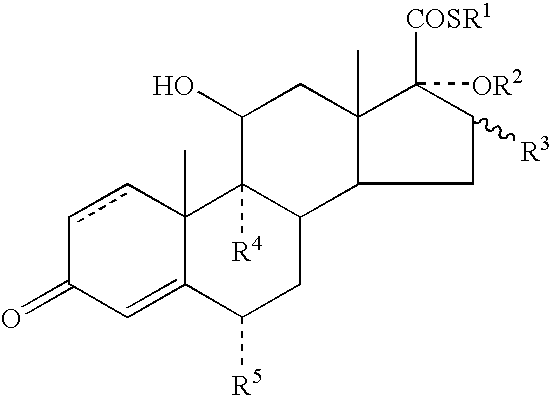
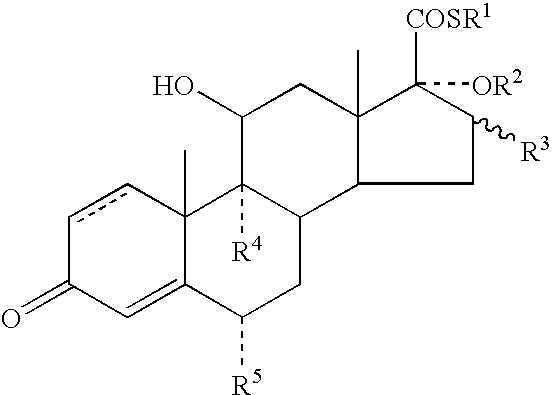
Dermatological Formulation
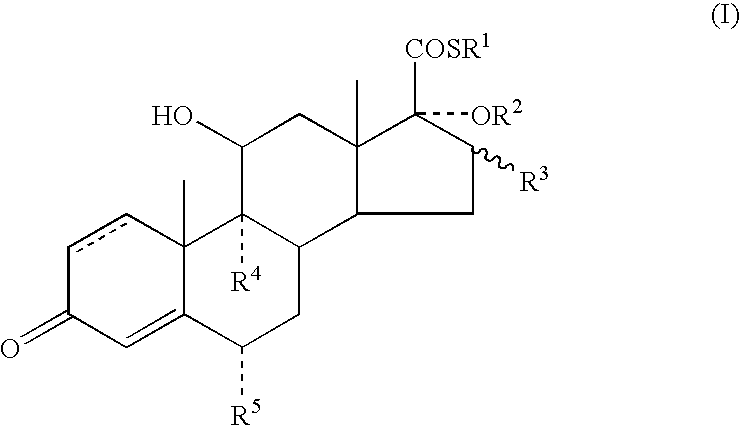
InactiveUS20030216364A1Reduce the amount of solutionImproved VC potencyOrganic active ingredientsCosmetic preparationsFluoroethylDouble bond
A topical formulation including a solvent, an occlusive agent, a surfactant system, an androstane steroid compound of formula (I) wherein R<1 >represents a fluoro-, chloro- or bromo-methyl group or a 2'-fluoroethyl group, R<2 >represents a group COR<6 >where R<6 >is a C1-3 alkyl group or OR<2 >and R<3 >together form a 16alpha, 17alpha-isopropylidenedioxy group; R<3 >represents a hydrogen atom, a methyl group (which may be in either the alpha- or beta-configuration) or a methylene group; R<4 >represents a hydrogen, chlorine or fluorine atom; R<5 >represents a hydrogen or fluorine atom and the symbol --- represents a single or double bond, and the balance being water.
Owner:SMITHKLINE BECKMAN CORP
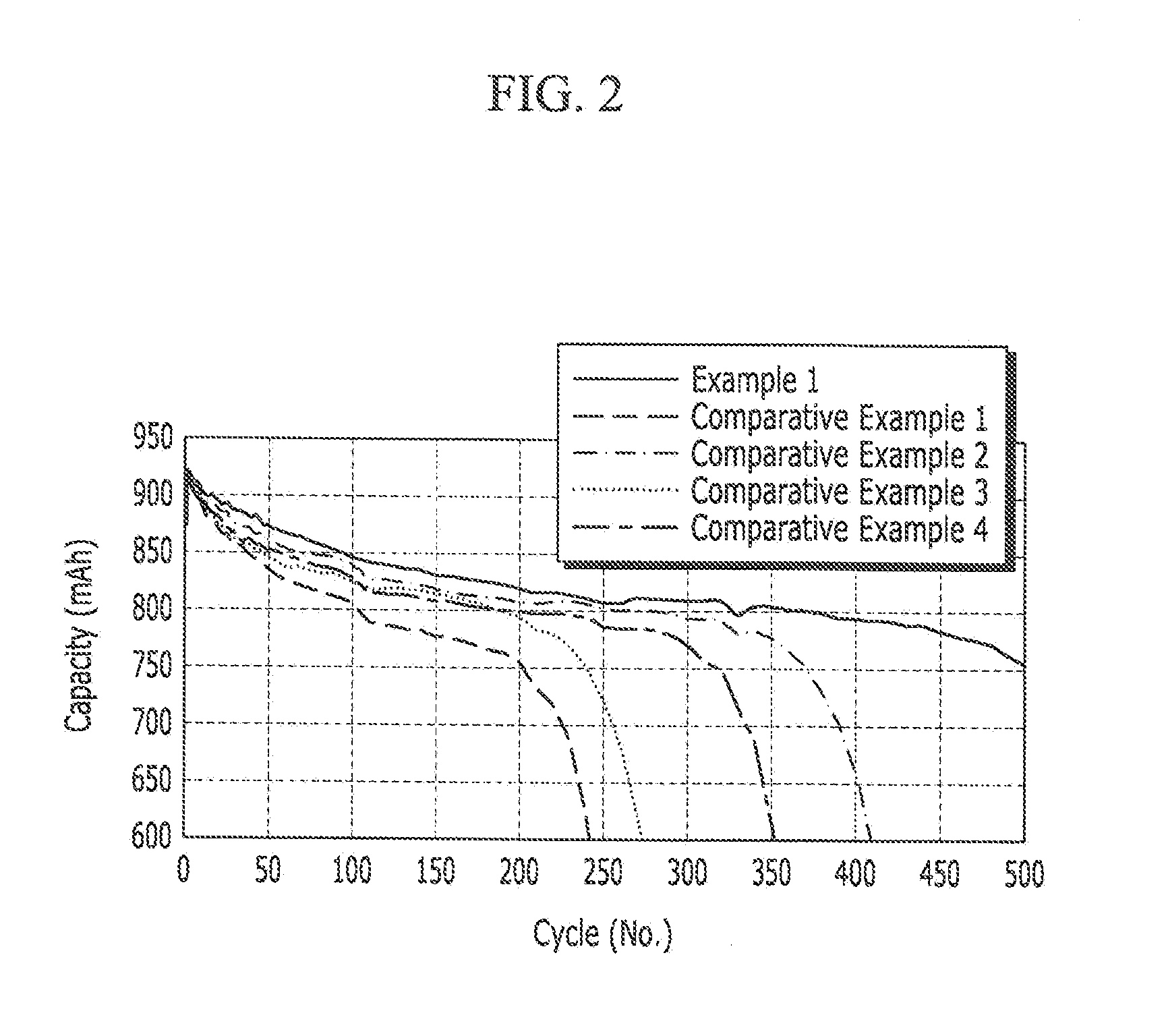
Electrolyte for rechargeable lithium battery, and rechargeable lithium battery including same
InactiveUS20120045697A1Inhibition of thickness increaseImproved cycle life characteristicsOrganic electrolyte cellsLi-accumulatorsOrganic solventFluoroethyl
Disclosed are an electrolyte for a rechargeable lithium battery that includes a lithium salt, a non-aqueous organic solvent, and an additive including a triazine-based compound represented by the following Chemical Formula 1 and fluoroethyl carbonate, and a rechargeable lithium battery including the electrolyte.where R1, R2, and R3 are the same as described in the detailed description.
Owner:SAMSUNG SDI CO LTD +1
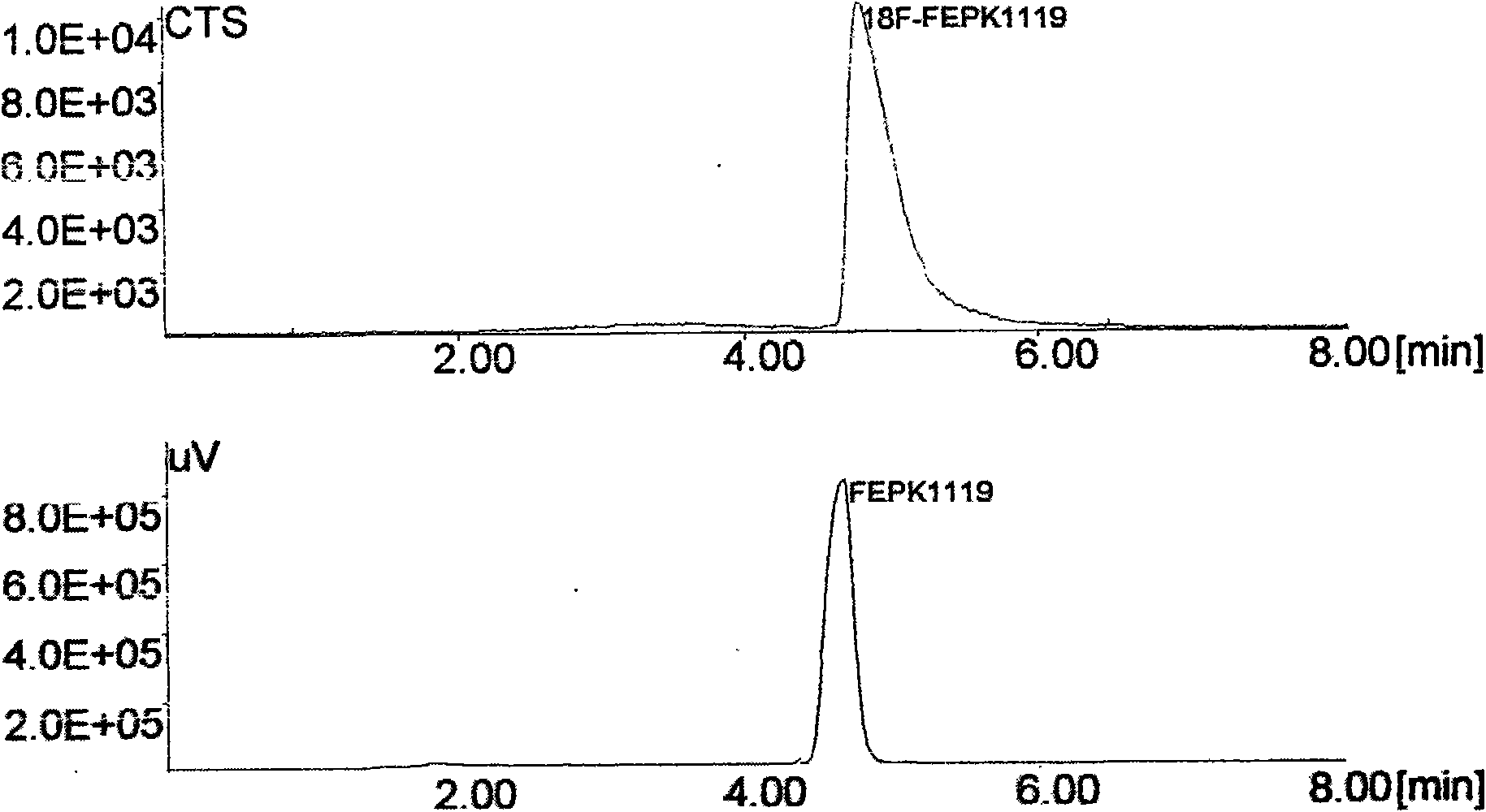
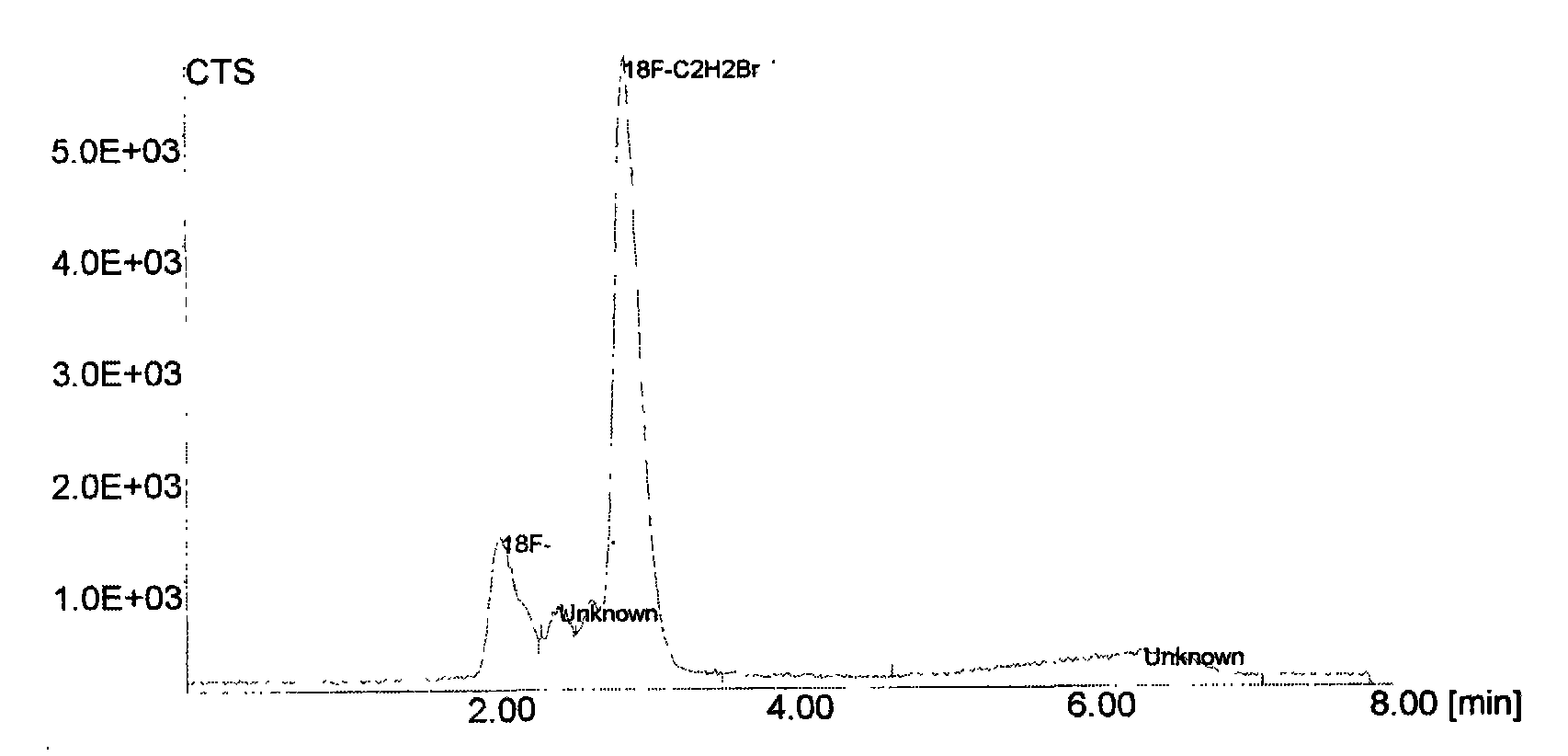
New synthetic method of PET imaging agent L-5-<18>FETP
InactiveCN101648899ASimple methodMethod stableOrganic chemistryRadioactive preparation carriersCarboxyl radicalEthylene glycol bis
The invention relates to a new synthetic method of a PET imaging agent L-5-<18>FETP. The method comprises the following steps: (a) carrying out a reaction between 5-hydroxytryptophan and C<1-3>alkylolto obtain a product; carrying out a reaction between the product and di-tert-butyl (i.e. (BOC)2O) to obtain N-BOC-L-5-hydroxytryptophan; (b) fluorizating 1,2-glycol tosylate in the presence of the ions such as potassium carbonate, Kryptate2.2.2 and <18>F<-> to obtain 2-<18F>-fluoroethanol p-toluene sulphonic acid ester (i.e. <18>FCH2CH2OTs); (c) carrying out a reaction between the 2-<18F>-fluoroethanol p-toluene sulphonic acid ester and the alkali metal salt of the N-BOC-L-5-hydroxytryptophan (such as sodium salt) to obtain O-(2-<18F>fluoroethyl)-N-BOC-L-5-hydroxytryptophan; (d) hydrolyzing the O-(2-<18F>fluoroethyl)-N-BOC-L-5-hydroxytryptophan under acidic conditions to remove an amino group and a carboxy protective group and obtain a final product of O-(2-<18F>fluoroethyl)-N-BOC-L-5-hydroxy tryptophan; and (e) purifying the product. The method greatly improves the radiochemical yield, obtains a sufficient quantity of a target product and realizes the clinical application of the target product.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
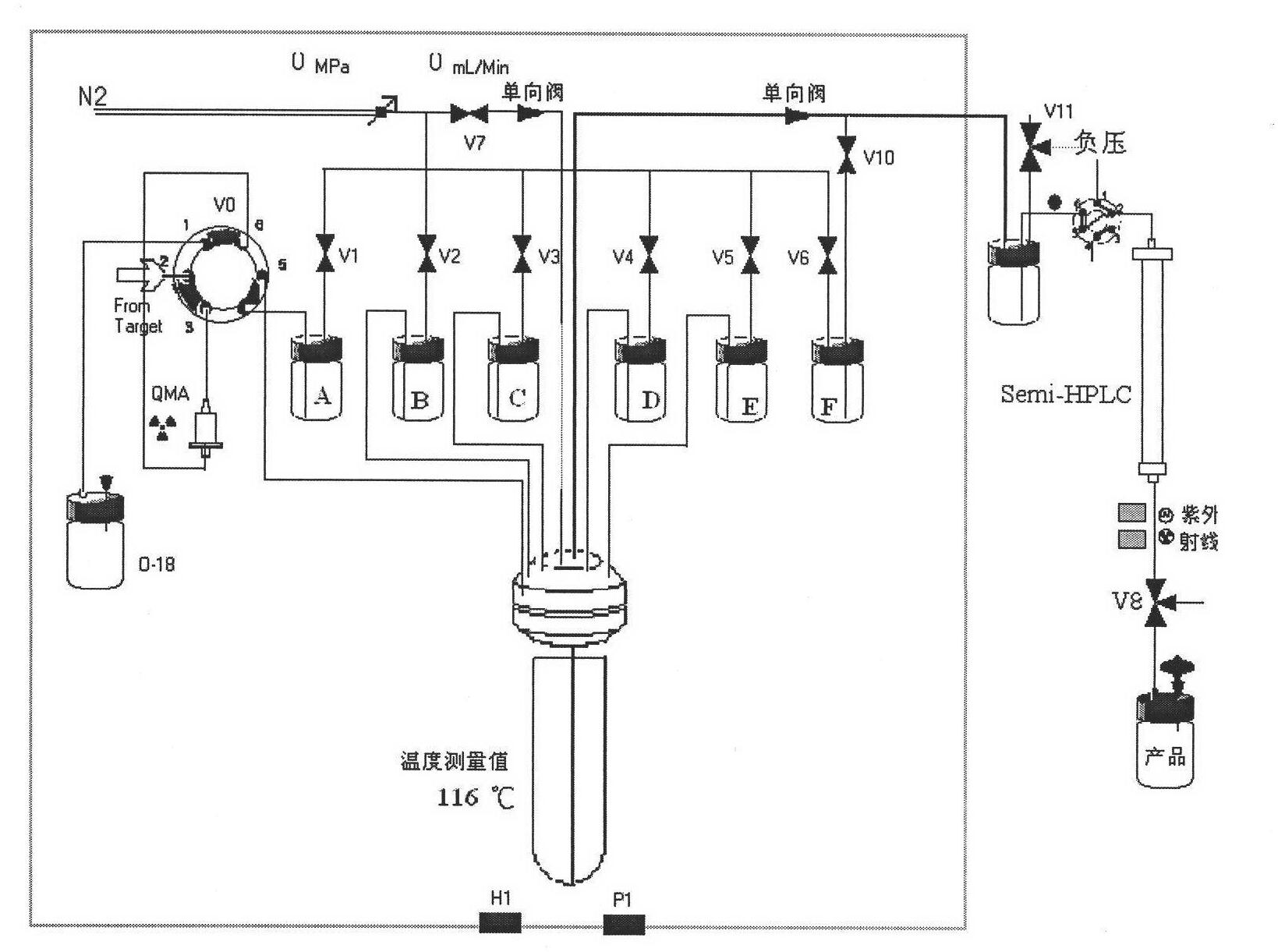
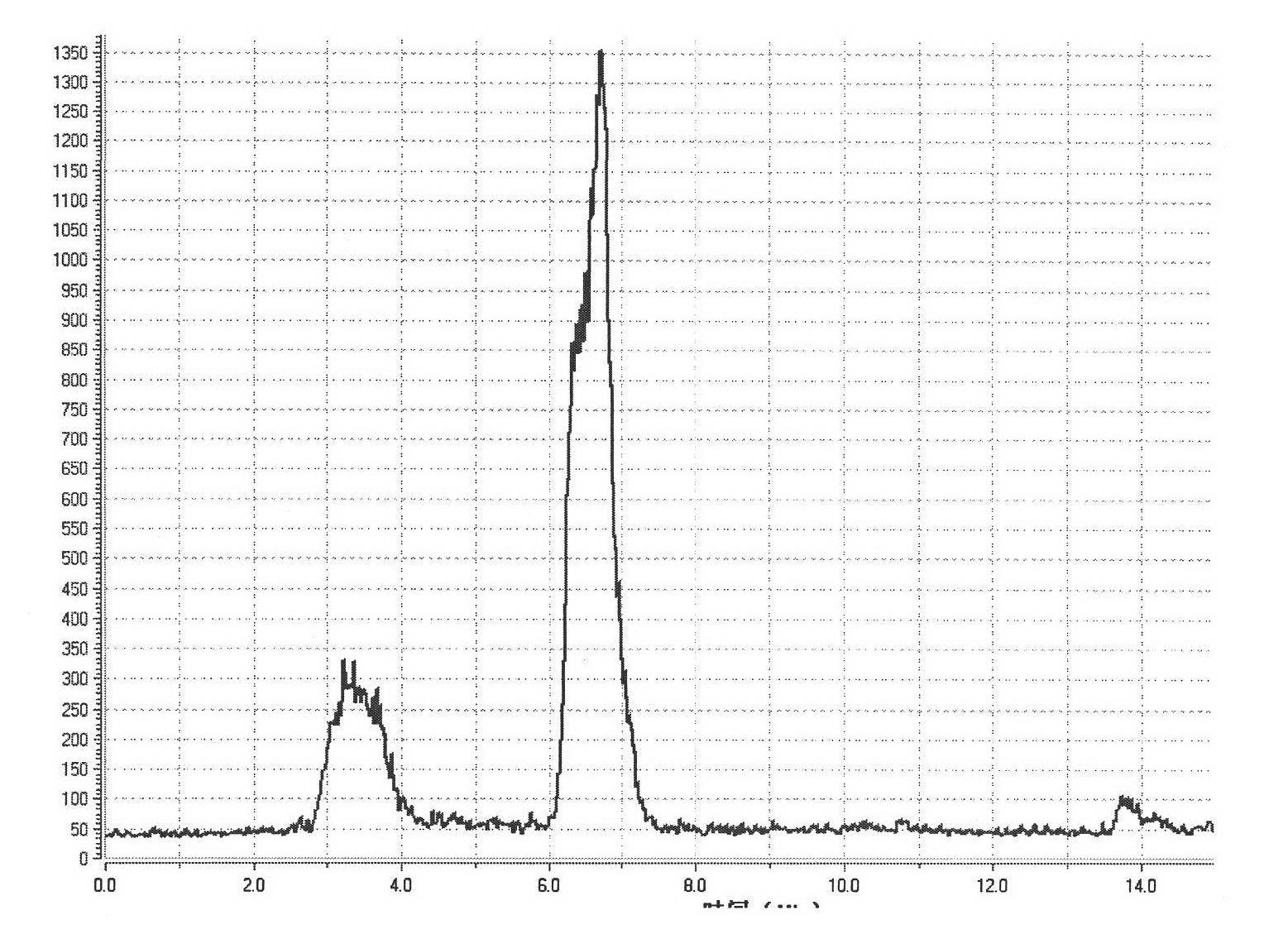
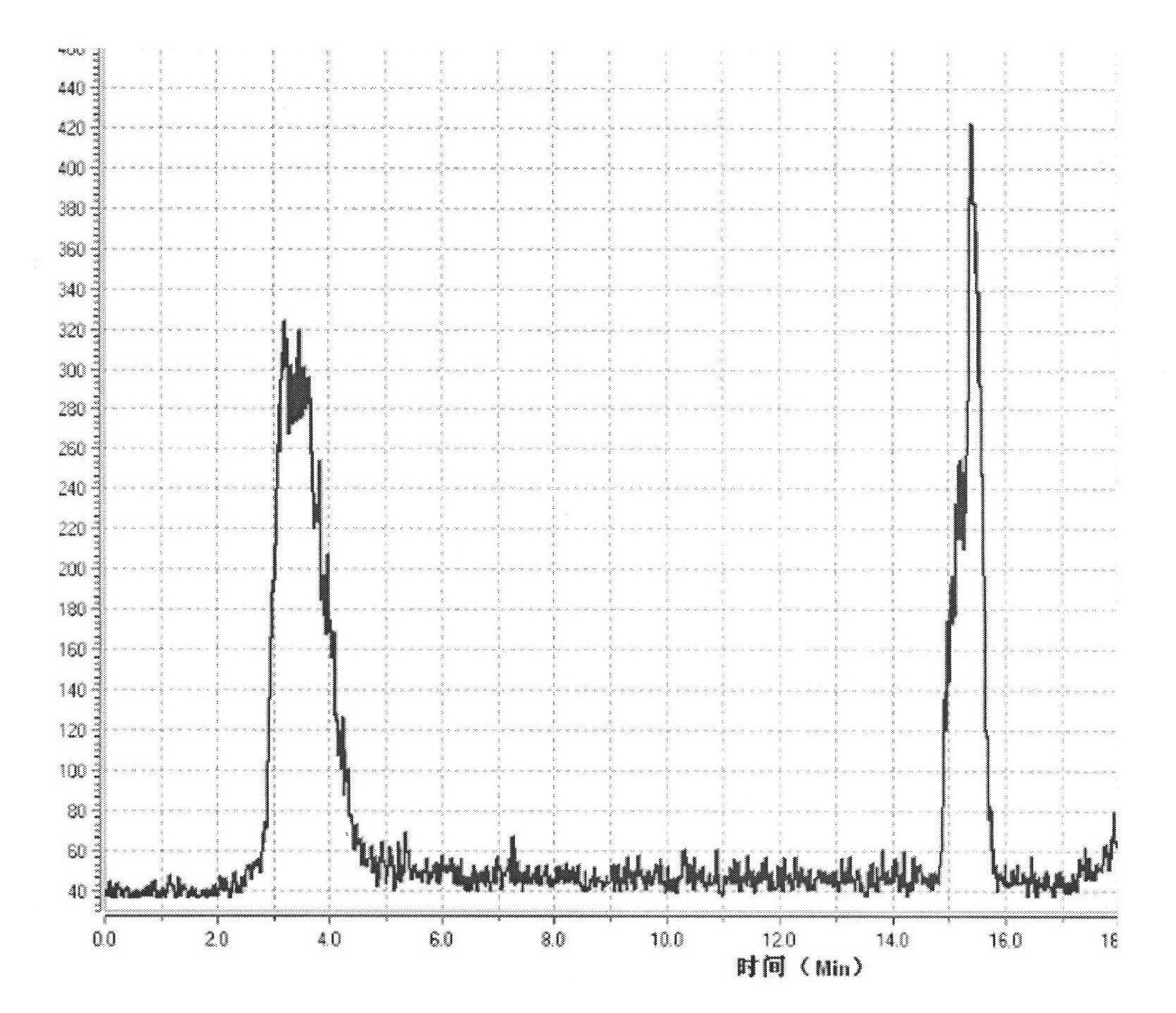
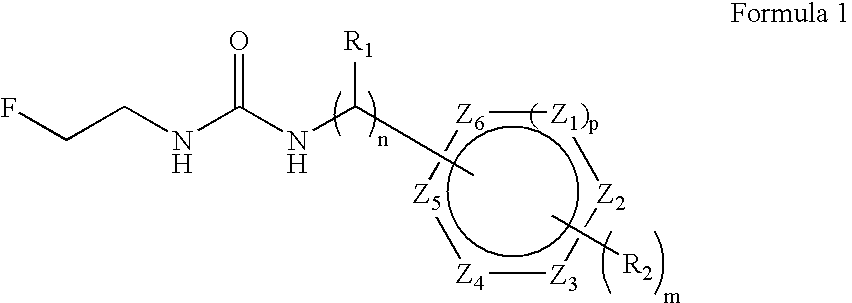
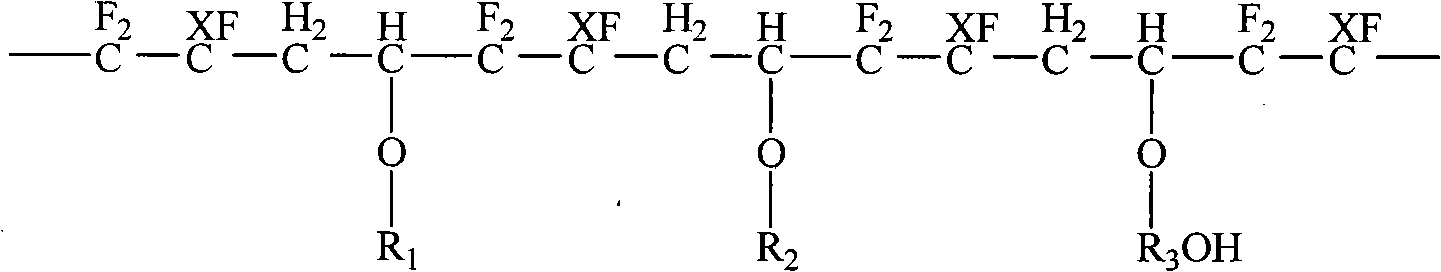
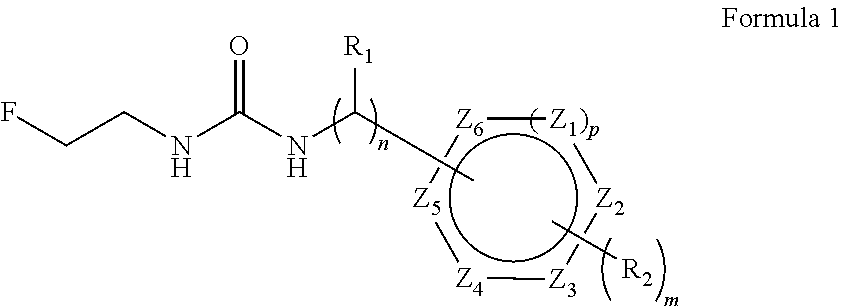
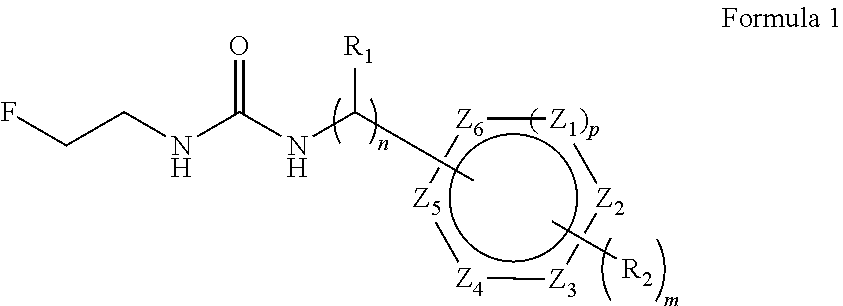
Convenient method for the preparation of new precursor of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine)
ActiveUS7138540B1Carbamic acid derivatives preparationOrganic compound preparationChemical structureLeaving group
This is a new precursor and new method for the synthesis of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine which has been proved a suitable PET (position emission tomography) probe for tumor diagnosis imaging, the preparation of the title compound starts from precursors with the chemical structures as in Formula 1, wherein R1 is a protective group for the carboxyl functional group, R2 is a protective group for the amino group, and R3 acts as a leaving group. R1 represents an arylalkyl group, R2 represents a carboxyl group, and R3 represents a p-tosyloxy, methane sulfonyloxy or trifluoromethane sulfonyloxy or bromine, the invention includes a method for the syntheses of new precursors with the chemical structures as in Formula 1.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Method for preparing 18F-FET
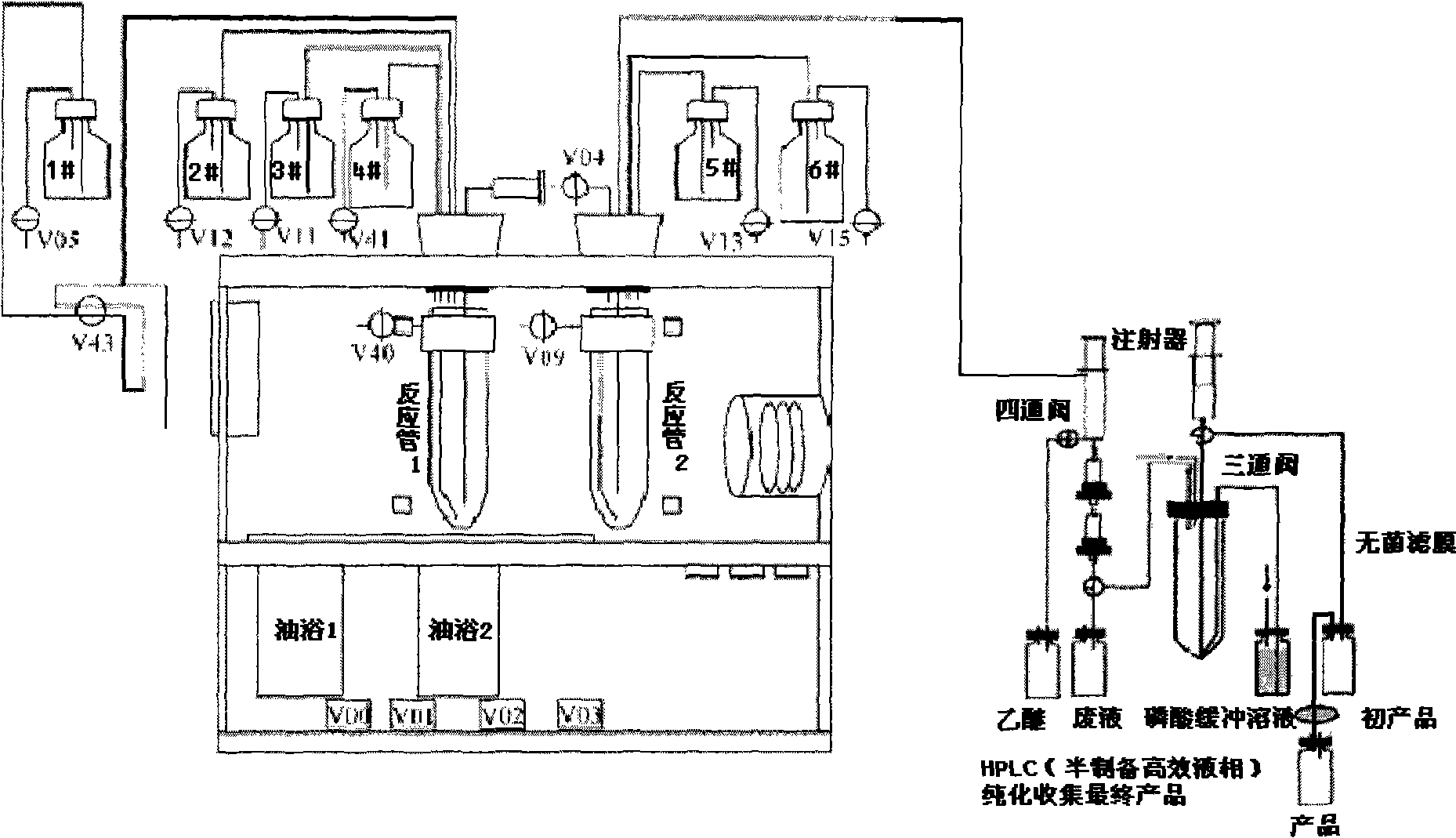
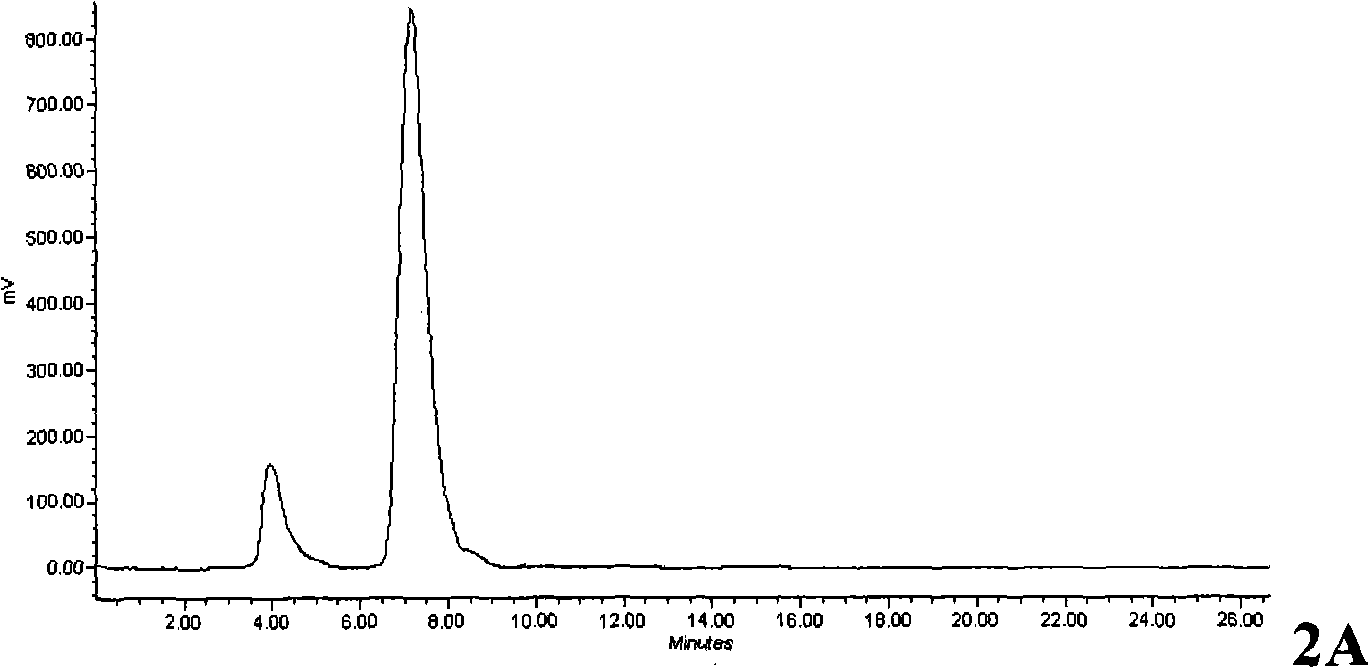
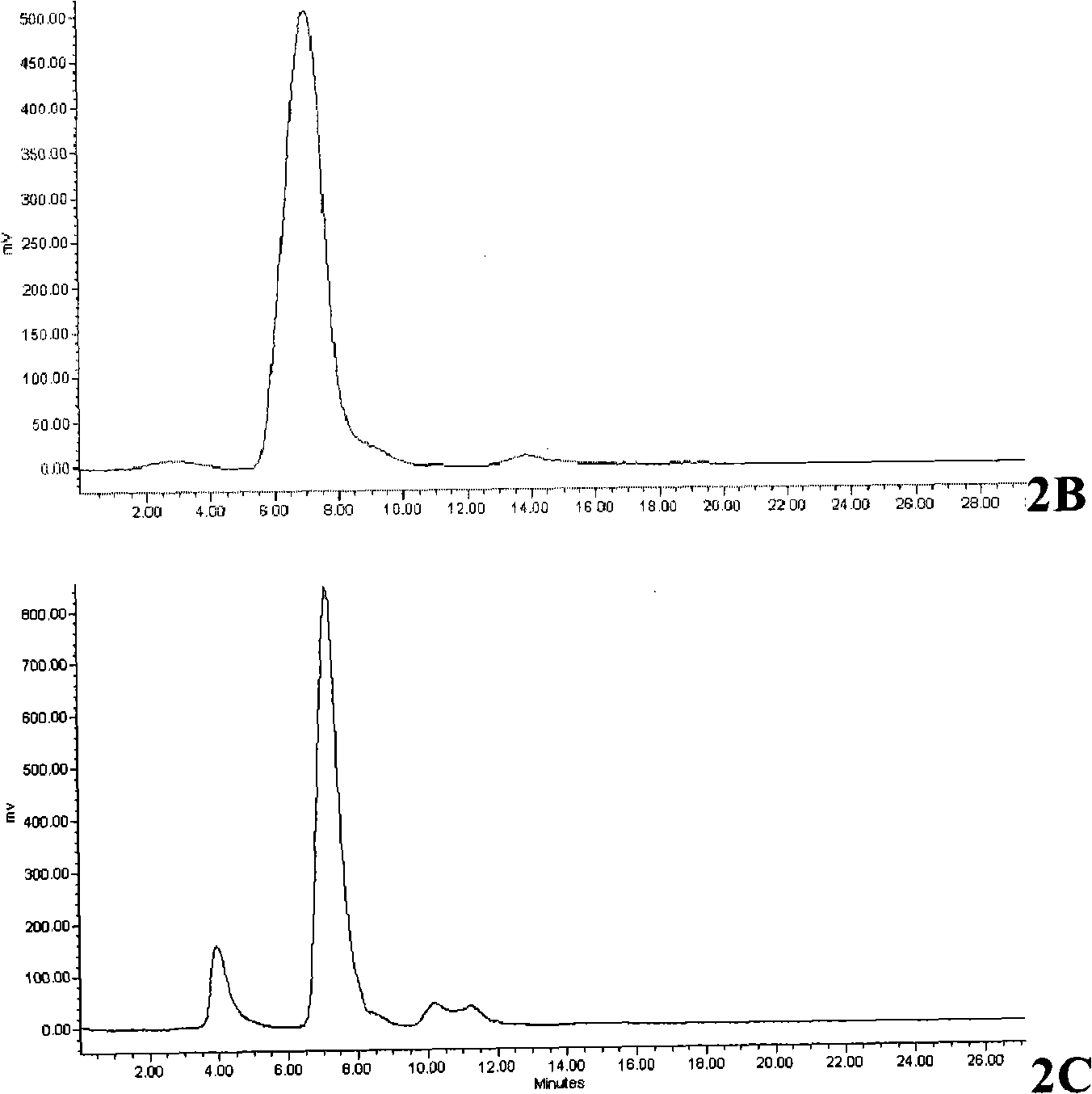
InactiveCN102336678AEliminate the need for purificationReduce productionOrganic compound preparationAmino-carboxyl compound preparationTyrosineFluoroethyl
The invention relates to a method for automatically synthesizing and purifying O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET). Ethylene glycol p-toluenesulfonate is used as a raw material and reacted with 18F-nucleophile on a fluorodeoxyglucose (FDG) module, the reaction product is not purified and is reacted with L-tyrosine salt in the same reactor, the product is purified by different mobile phases of high performance liquid chromatography (HPLC), and the separated product is neutralized and then filtered by a sterile filtration membrane to meet the injection requirement. The process can be automatically operated on the common FDG module, and has the advantages of short synthesis time (30 minutes, comprising HPLC purification) and high yield (40 percent, correction).
Owner:GENERAL HOSPITAL OF PLA +1
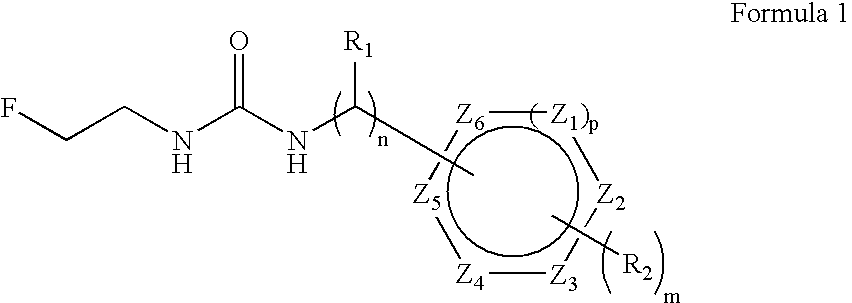
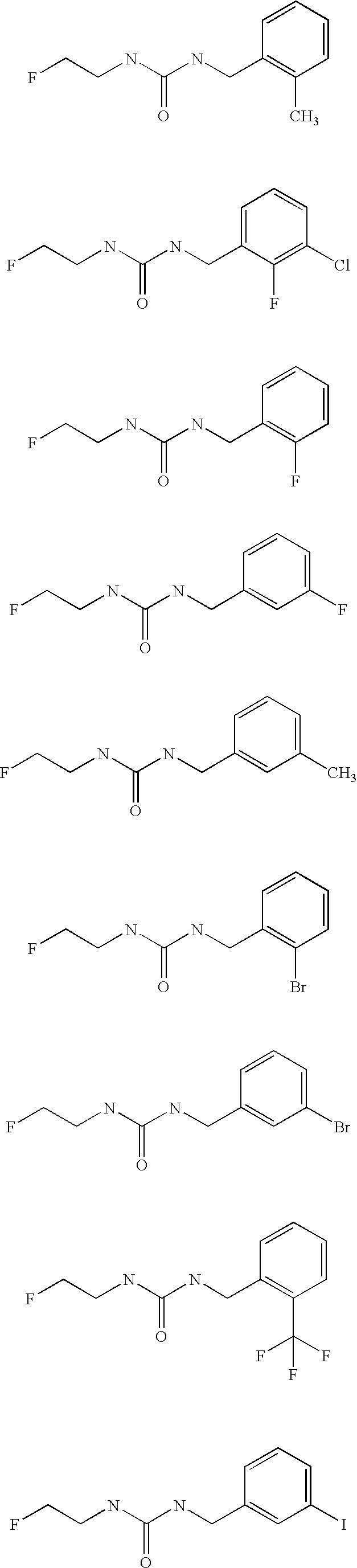
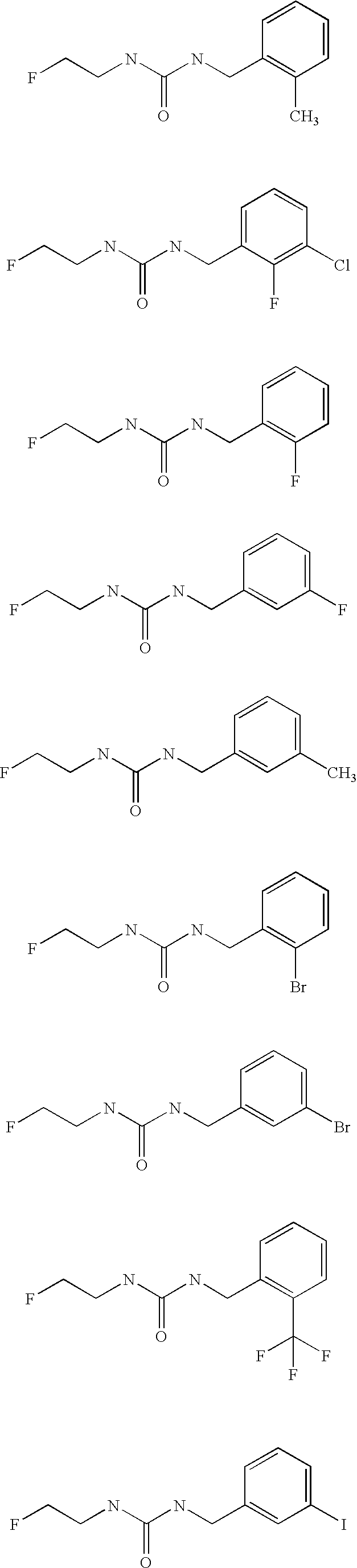
Aryl fluoroethyl ureas acting as alpha 2 adrenergic agents
The invention provides well-defined aryl fluoroethyl ureas that are useful as selective alpha2 adrenergic agonists. As such, the compounds described herein are useful in treating a wide variety of disorders associated with modulation of alpha2 adrenergic receptors.
Owner:ALLERGAN INC
Positron emission tomography (PET) diagnostic radioactive drug and preparation method thereof
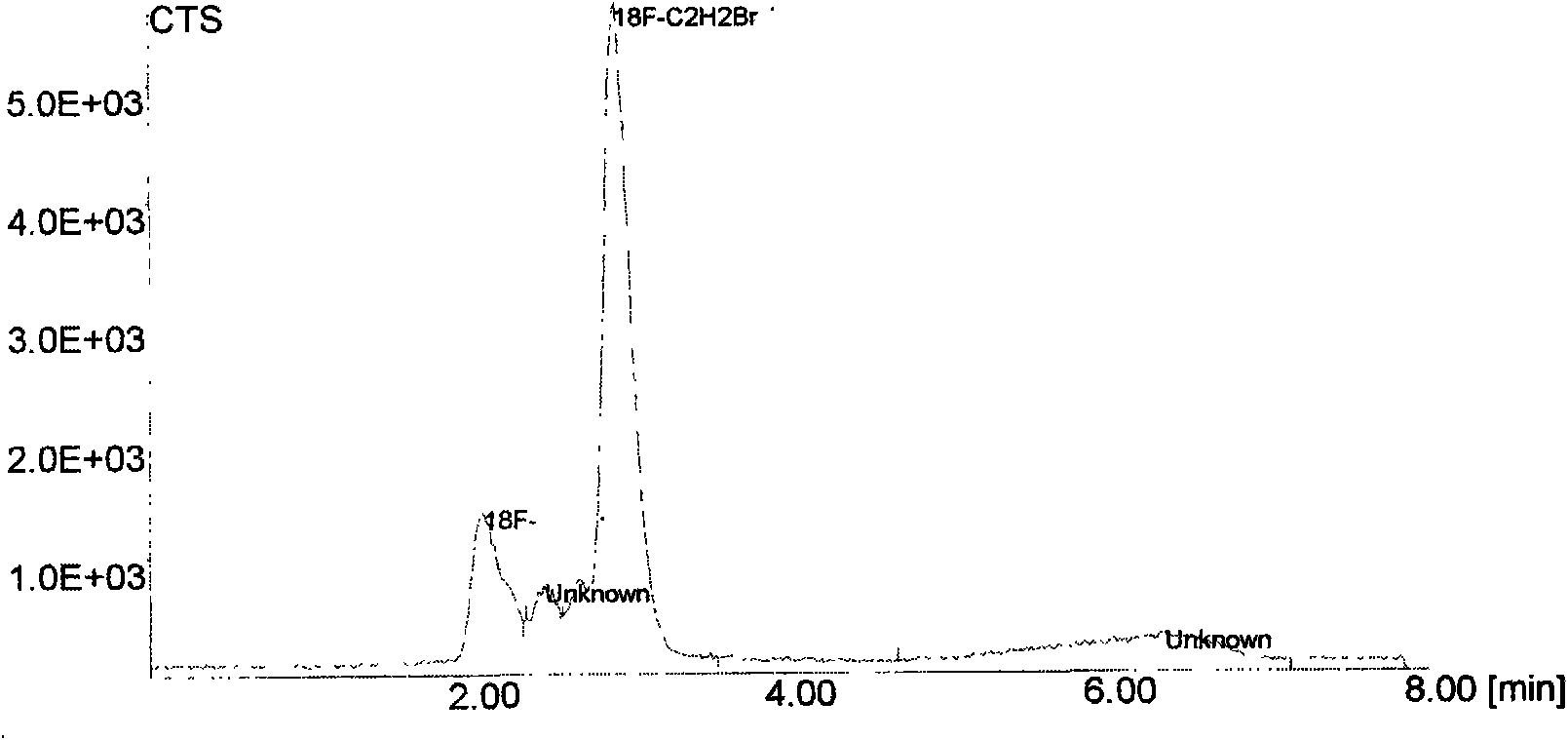
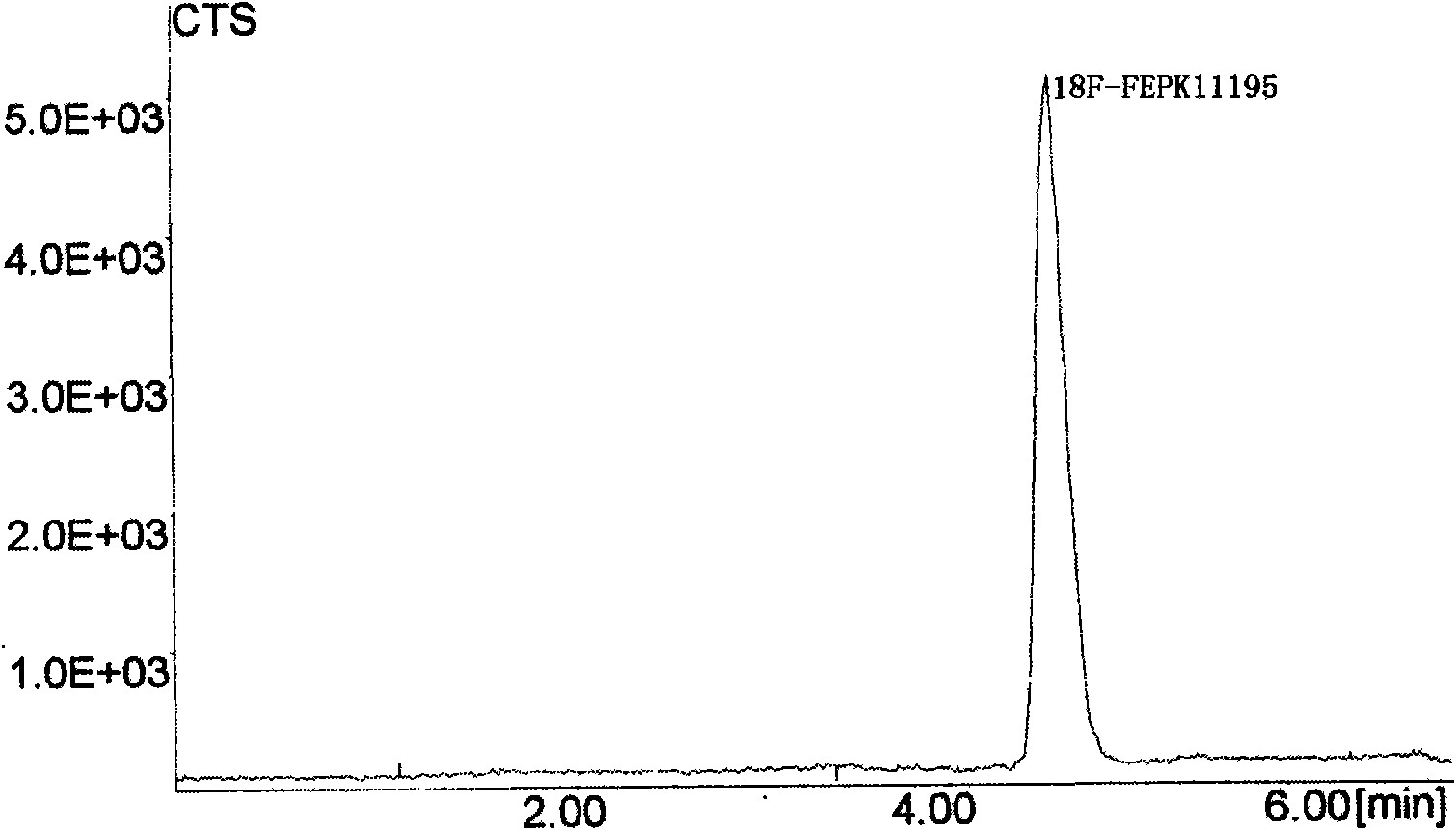
ActiveCN101648027AIncreased steric hindranceReduces in vivo degradationIn-vivo radioactive preparationsChlorobenzeneRadioactive drug
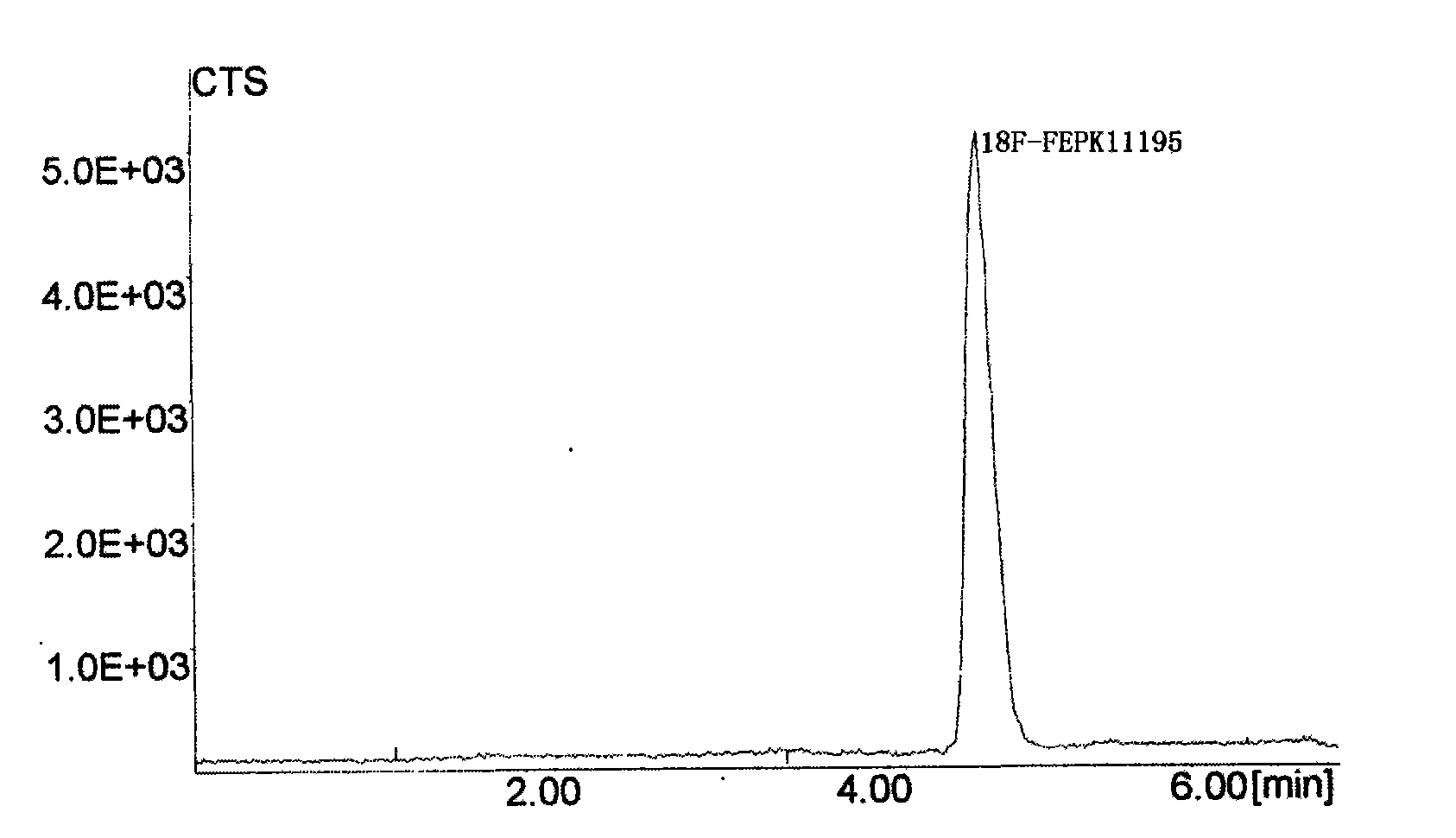
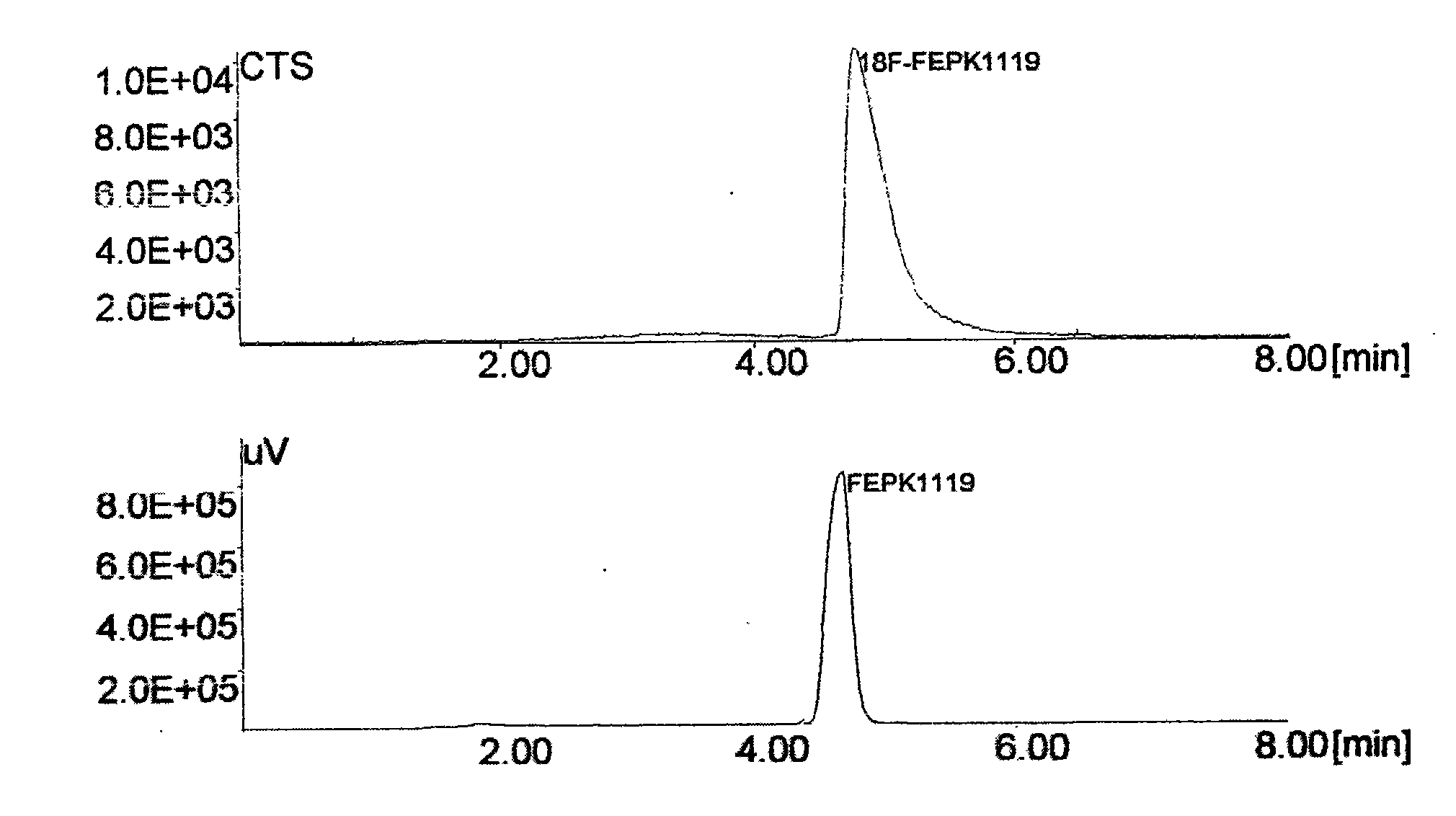
The invention discloses a positron emission tomography (PET) diagnostic radioactive drug, N-[<18>F]fluoroethyl-N-(1-methylpropyl)-1-(2-chlorophenyl)isoquinoline-3-carbamoyl. The preparation method comprises the following steps: using <18>F<-> and 1,2-dibromoethane to react and prepare labeling intermediate 1-bromo-2-[<18>F]fluoroethane, then using 1-bromo-2-[<18>F]fluoroethane and precursor compound nor-PK11195 to perform alkylation reaction and obtain the finished product. In the method of the invention, [<18>F]ethyl structure is introduced in the molecule of PK11195 so as to increase the space steric effect of molecules, reduce the degradation in vivo of ligand molecules and further increase the signal-to-noise ratio of PET and sensitivity in the detection zone and effectively cover theshortage of the existing PET diagnostic radioactive drug.
Owner:GUANGDONG HUIXUAN PHARMA TECH
Fluorine-containing network structure ion exchange membrane based on fluoroethyl vinyl ether polyalcohol and preparation method thereof
InactiveCN101791526AGood effectImprove conductivitySemi-permeable membranesSecondary cellsCross-linkVinyl ether
The invention relates to a fluorine-containing network structure ion exchange membrane based on fluoroethyl vinyl ether polyalcohol and preparation method thereof. Polar organic solvent is adopt to dissolve perfluorinated sulfonic acid resin and anhydrous poly isocyanate as well as anhydrous fluoroethyl vinyl ether (FEVE) polyalcohol; tape casting is adopted to form a membrane on smooth solid surface; then heating is carried out and poly isocyanate and anhydrous FEVE take polymerization, and products of the polymerization and perfluorinated sulfonic acid molecule chain form ion exchange membrane in macromolecule interpenetrating network structure. The membrane preparation method can obtain fluorine-containing ion exchange membrane material with favourable proton commutativity, overcomes the defect that the existing fusion mould pressing process can not prepare homogeneous cross linking ion exchange membrane and has the advantages of simple technological process and easy industrial scale-up.
Owner:SHANDONG DONGYUE POLYMER MATERIAL
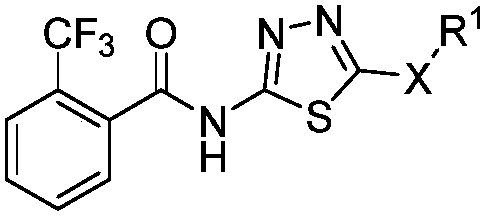
1,3,4-thiadiazole thioether (sulfone)-containing 2-(trifluoromethyl)benzamide derivative and preparation and application thereof
ActiveCN109456283APrevention and treatment of co-infectionBiocideOrganic chemistryDiseaseFluoroethyl
The invention discloses a 1,3,4-thiadiazole thioether (sulfone)-containing 2-(trifluoromethyl)benzamide derivative and preparation and application thereof. A general formula of the 1,3,4-thiadiazole thioether (sulfone)-containing 2-(trifluoromethyl)benzamide derivative is as shown in the description, wherein R1 is methyl or ethyl or 2-chloroethyl or 2-fluoroethyl or 2-bromomethyl or propyl or butyl or amyl or 4-cyanobenzyl or 4-chlorobenzyl or 2-fluorobenzyl or cyanomethyl or 2-cyanoethyl or 3-cyanopropy; X is S or -S(O)2-. The 1,3,4-thiadiazole thioether (sulfone)-containing 2-(trifluoromethyl)benzamide derivative can control compound infection of meloidogyne incognita and rice bacterial leaf blight, meloidogyne incognita and tobacco bacterial wilt, and meloidogyne incognita and citrus bacterial canker disease.
Owner:GUIZHOU UNIV
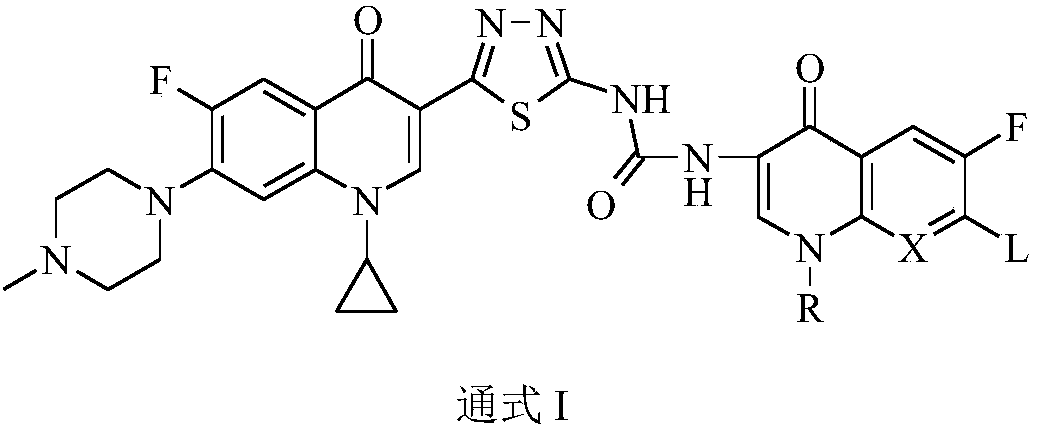
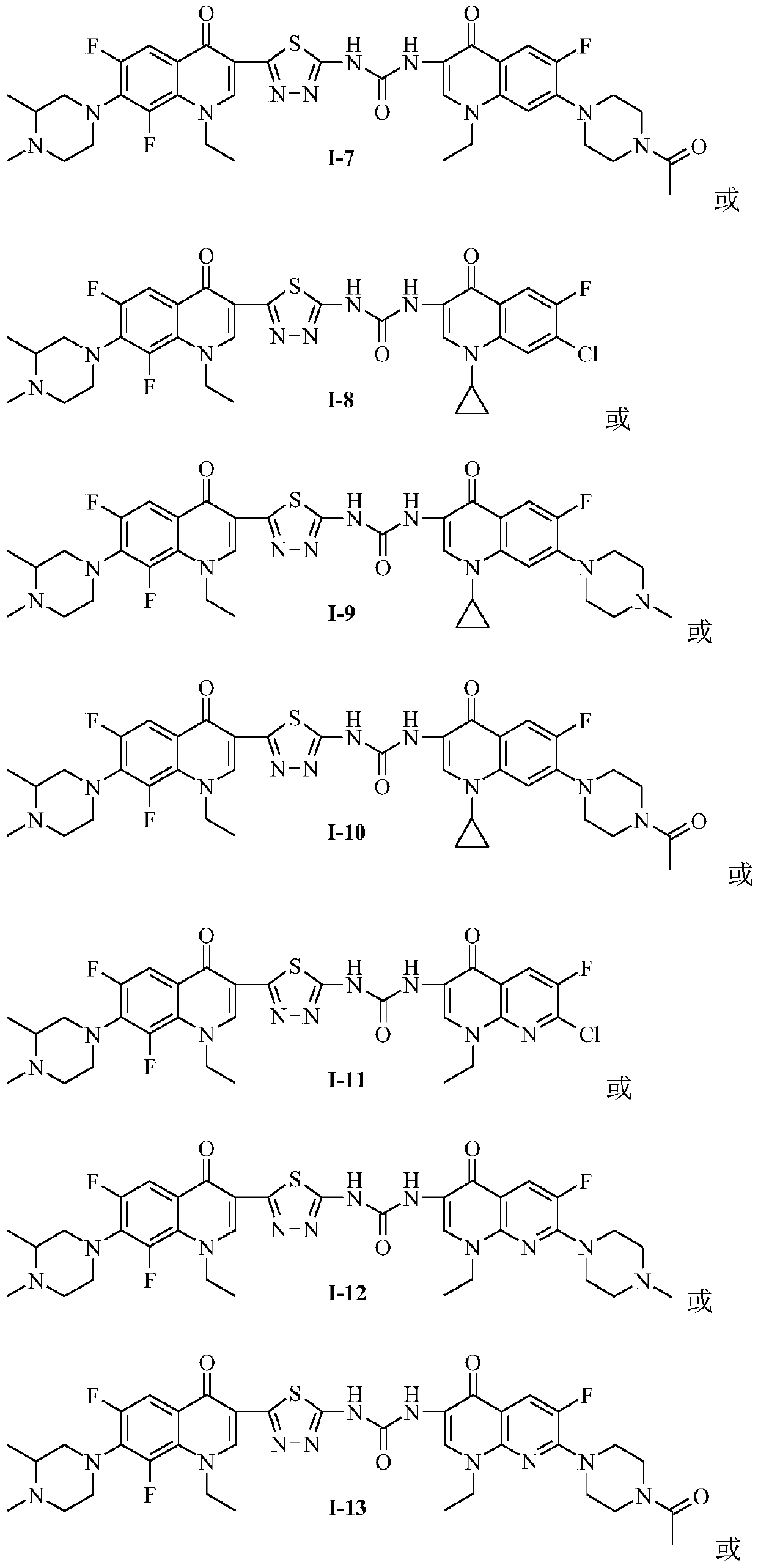
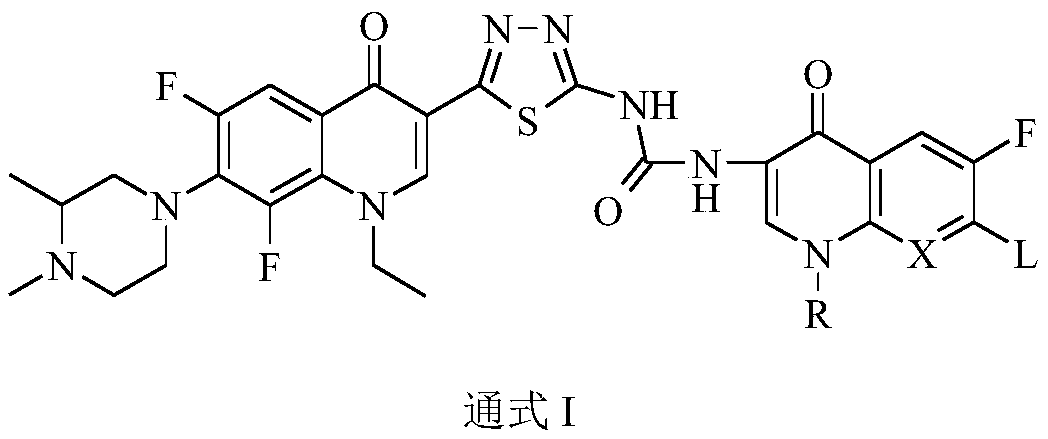
Preparation and application of bis-fluoroquinolone thiadiazole ureas N-methyl ciprofloxacin derivative
PendingCN109678884AStructural innovationAchieve migrationOrganic chemistryAntineoplastic agentsFluoroethylEthyl Chloride
The invention discloses a bis-fluoroquinolone thiadiazole ureas N-methyl ciprofloxacin derivative and a preparation method and application thereof. The chemical structural general formula of the bis-fluoroquinolone thiadiazole ureas N-methyl ciprofloxacin derivative is shown in the following formula I (shown in the specification), according to the formula I, R is ethyl or cyclopropyl or fluoroethyl or an oxazine ring formed with a C-8 position or a thiazine ring formed with the C-8 position, L is independent chlorine atom or fluorine atom or 1-piperazinyl or substituted piperazine-1-yl or a nitrogen fused heterocyclic ring, and X is hydrocarbon (CH) or nitrogen atom or fluorine-substituted carbon atom (F-C) or methoxy-group-substituted carbon atom (CH3O-C). According to the bis-fluoroquinolone thiadiazole ureas N-methyl ciprofloxacin derivative and the preparation method and application thereof, organic combination of a bis-fluoroquinolone skeleton, the thiadiazole heterocyclic ring and functional group urea is achieved, the charge transfer and superposition of different pharmacophore are further achieved, the anti-tumor activity and selectivity of fluoroquinolone are increased, toxic and side effects on normal cells are reduced, and the bis-fluoroquinolone thiadiazole ureas N-methyl ciprofloxacin derivative can be used as anti-tumor active substances to develop anti-tumor drugs with brand-new structures.
Owner:HENAN UNIVERSITY
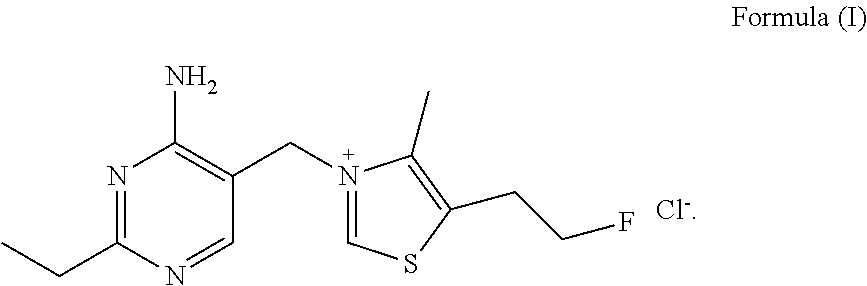
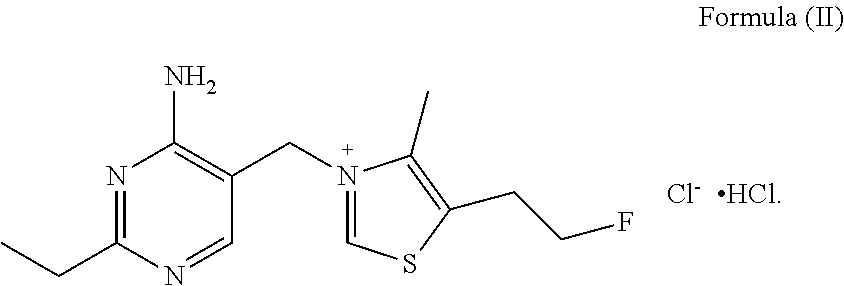
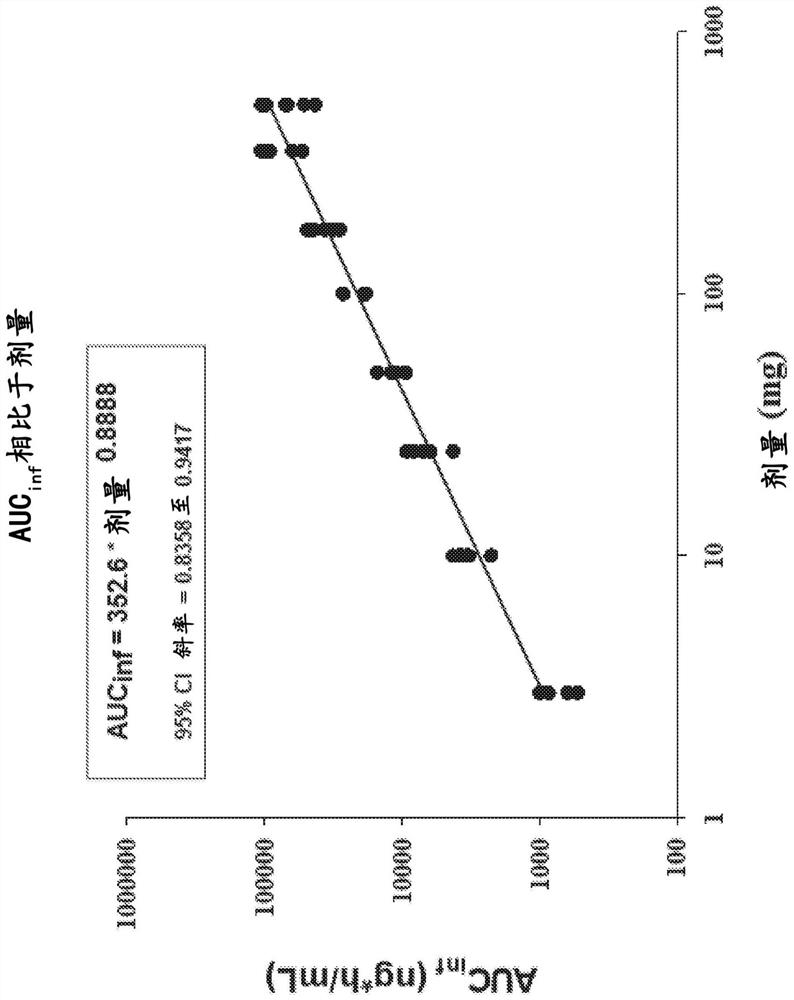
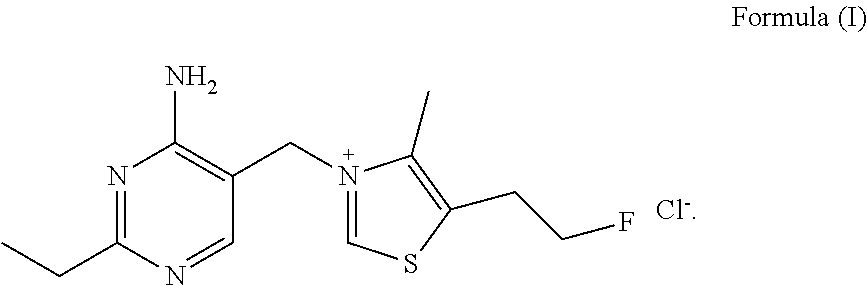
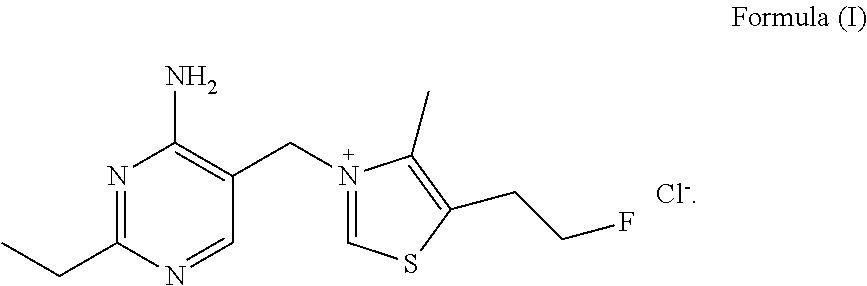
Fluoroethyl thiamine or salts thereof and application thereof in preparation of anticoccidial drugs
The present invention discloses a fluoroethyl thiamine or salts thereof and application thereof in preparation of anticoccidial drugs. The structural formula of the fluoroethyl thiamine or salts thereof is shown as Formula (I). The fluoroethyl thiamine or salts thereof of the present invention have a remarkable anticoccidial effect, particularly on some coccidia which had resistance to other anticoccidial drugs, therefore the fluoroethyl thiamine or salts thereof of the present invention can be applied to preparation of anticoccidial drugs. Thus, the present invention provides conditions for development of new anticoccidial drugs.
Owner:GUANGZHOU INSIGHER BIOTECHNOLOGY CO LTD
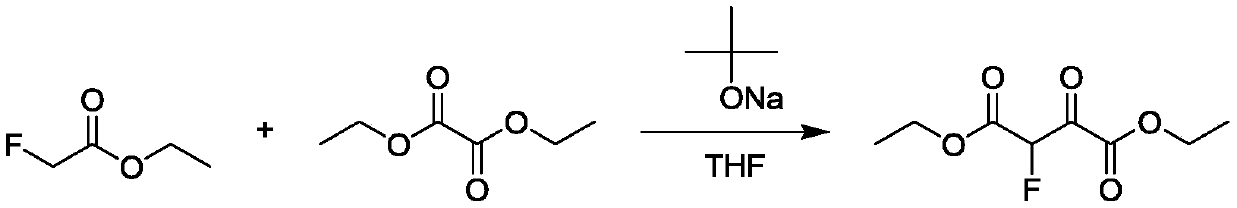
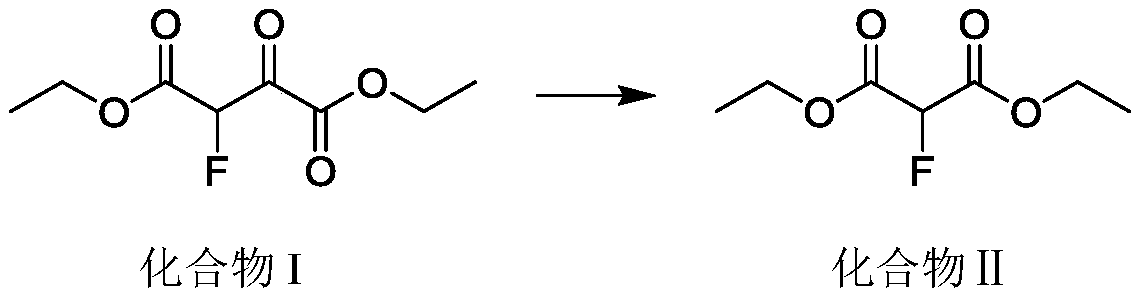
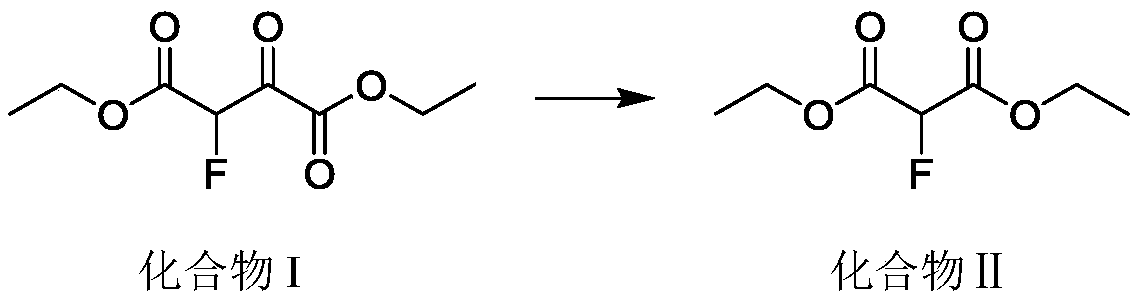
Continuous synthesis method for 2-fluoroethyl malonate compound
ActiveCN110437069AAvoid decompositionEasy to controlOrganic compound preparationCarboxylic acid esters preparationArylSynthesis methods
The invention discloses a continuous synthesis method for a 2-fluoroethyl malonate compound. The continuous synthesis method comprises the following steps: taking a formula shown in the description asa raw material, and carrying out a continuous decarbonylation reaction in continuous reaction equipment, thereby obtaining the 2-fluoroethyl malonate compound represented by a formula shown in the description, wherein R and R' separately represent straight-chain or branched alkyl, substituted or unsubstituted aryl or substituted or unsubstituted heterocycle or cyclic alkyl and are the same or different. According to the method, the high-temperature decarbonylation reaction of 2-fluoro-3-oxo-disuccinate is achieved by adopting the continuous reaction equipment. Compared with the traditional pot type reaction, the amount of materials participating in reaction in unit time is reduced greatly, so that a high-temperature hazardous area is reduced, and the security risk is greatly lowered.
Owner:ASYMCHEM LIFE SCI TIANJIN
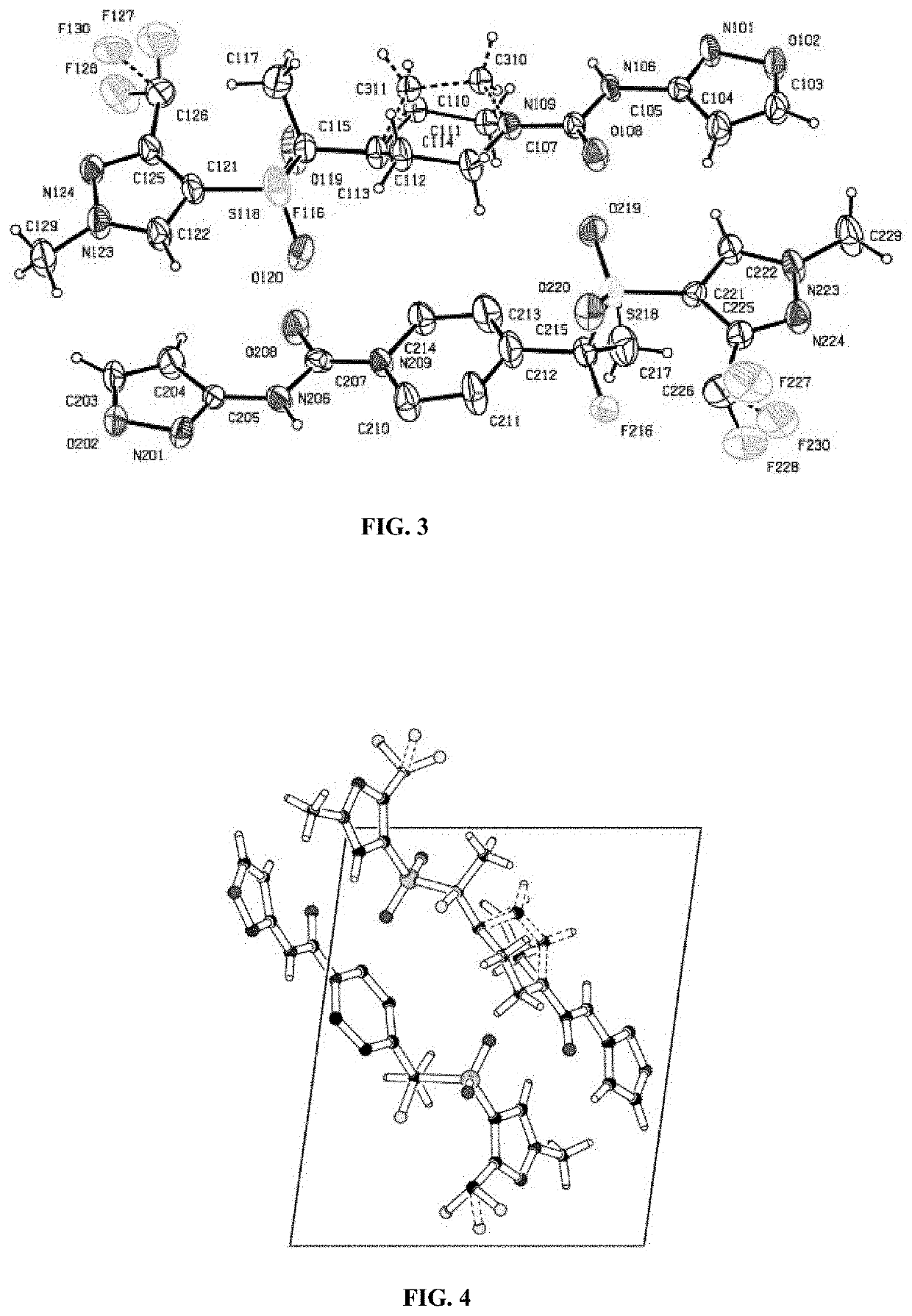
7-{(3S,4S)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidine-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal
ActiveCN103930414AImprove solubilityGood storage stabilityAntibacterial agentsOrganic active ingredientsSolubilityDecomposition
The purpose of the present invention is to provide a hydrochloride crystal, a hydrochloride hydrate crystal, and a methanesulfonate crystal of the compound represented by formula (1). These crystals are less susceptible to decomposition caused by the effects of light, and also have high preservation stability and high water solubility compared to a free crystal of the compound (1).
Owner:KYORIN PHARMA CO LTD
3,3'-methylene-bisfluoroquinolone derivative containing ethylquinoline rings as well as preparation method and application thereof
InactiveCN104447537ASmall toxicityAchieve overlayOrganic chemistryAntineoplastic agentsQuinolonePharmacophore
The invention discloses a 3,3'-methylene-bisfluoroquinolone derivative containing ethylquinoline rings as well as a preparation method and application thereof. The 3,3'-methylene-bisfluoroquinolone derivative containing ethylquinoline rings is a compound having the following structural general formula (I) as shown in the specification, wherein R1 is ethyl, cyclopropyl or fluoroethyl; R2 is H or methyl; R3 is H, methyl or ethyl; R4 is H or methyl; and X is CH, N, F-C or CH3O-C. The preparation method comprises the following steps of effectively combining fluoroquinolone pharmacophores and alpha, beta-unsaturated ketone pharmacophores and constructing two quinolone structural units into the 3,3'-methylene-bisfluoroquinolone derivative by virtue of condensation reaction, the anti-tumor activity is increased, the toxic and side effects to normal cells are decreased, the synergistic and toxicity-reducing effects are achieved and the 3,3'-methylene-bisfluoroquinolone derivative can be used as an anti-tumor active material to develop anticancer drugs having novel structures.
Owner:HENAN UNIVERSITY
Convenient method for the preparation of no-carrier-added O-(2-[18F]fluoroethyl)-L-tyrosine)
ActiveUS7132563B1Lower synthesis costEasy to operateCarbamic acid derivatives preparationOrganic compound preparationChemical structureLeaving group
This is a novel method for production of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine, which has been proved a suitable PET (position emission tomography) probe for tumor diagnosis imaging, and the preparation of the title compound starts from precursors with the chemical structures as in Formula 1, wherein R1 is a protective group for the carboxyl functional group, R2 is a protective group for the amino group, and R3 acts as a leaving group, R1 represents an arylalkyl group, R2 represents a carboxyl group, and R3 represents a p-tosyloxy, methane sulfonyloxy or trifluoromethane sulfonyloxy or bromine, and the final purification of the product is using a separation column, which is very convenient for automated synthesis, and the invention uses the precursor with the chemical structures as in Formula 1.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Bi-fluoroquinolone oxadiazole ureas N-acetyl norfloxacin derivative and preparation method and application thereof
InactiveCN109369676AStructural innovationAchieve migrationOrganic active ingredientsOrganic chemistrySide effectPharmacophore
The invention discloses a bi-fluoroquinolone oxadiazole ureas N-acetyl norfloxacin derivative and a preparation method and application thereof. The bi-fluoroquinolone oxadiazole ureas N-acetyl norfloxacin derivative is shown as the formula (Please please seed the specifications for the formula), wherein R is an oxazine ring constituted by ethyl, cyclopropyl, fluoroethyl and C-8 potential or is a thiazine ring constituted by the ethyl, the cyclopropyl, the fluoroethyl and the C-8 potential; L is independent helium atoms, fluorine atoms, 1-piperazinyl, substituted piperazine-1-yl or nitrogen fused heterocycle; and X is -CH (carbon hydrogen), N (notrigen atoms), -CF (fluorine-substituted carbon atoms) or -COCH3 (methoxy-substituted carbon atoms). According to the bi-fluoroquinolone oxadiazoleureas N-acetyl norfloxacin derivative, a bi-fluoroquinolone skeleton, oxadiazole heterocycle and functional base ureas are organically spliced, thus transferring and overlaying of different pharmacophore are achieved, the antitumor activity and selectivity of fluoroquinolone are improved, the toxic and side effects onof normal cells are lowered, and the bi-fluoroquinolone oxadiazole ureas N-acetyl norfloxacin derivative can be used as an antitumor active substance for developing an antitumor drug of a brand-new structureas an antitumor drug of antitumor active substance development brand-newstructure.
Owner:ZHENGZHOU UNIV OF IND TECH
Aryl fluoroethyl ureas acting as alpha 2 adrenergic agents
The invention provides well-defined aryl fluoroethyl ureas that are useful as selective alpha2 adrenergic agonists. As such, the compounds described herein are useful in treating a wide variety of disorders associated with modulation of alpha2 adrenergic receptors.
Owner:ALLERGAN INC
Preparation and application of bis-fluoroquinolone thiadiazole ureas N-acetyl norfloxacin derivative
InactiveCN109678883AStructural innovationAchieve migrationOrganic chemistryAntineoplastic agentsSide effectPharmacophore
The invention discloses a bis-fluoroquinolone thiadiazole ureas N-acetyl norfloxacin derivative and a preparation method and application thereof. The chemical structural general formula of the bis-fluoroquinolone thiadiazole ureas N-acetyl norfloxacin derivative is shown in the following formula I (shown in the specification), according to the formula I, R is ethyl or cyclopropyl or fluoroethyl oran oxazine ring formed with a C-8 position or a thiazine ring formed with the C-8 position, L is independent chlorine atom or fluorine atom or 1-piperazinyl or substituted piperazine-1-yl or a nitrogen fused heterocyclic ring, and X is hydrocarbon (CH) or nitrogen atom or fluorine-substituted carbon atom (F-C) or methoxy-group-substituted carbon atom (CH3O-C). According to the bis-fluoroquinolonethiadiazole ureas N-acetyl norfloxacin derivative and the preparation method and application thereof, organic combination of a bis-fluoroquinolone skeleton, the thiadiazole heterocyclic ring and functional group ureas is achieved, the charge transfer and superposition of different pharmacophore are further achieved, the anti-tumor activity and selectivity of fluoroquinolone are increased, toxic and side effects on normal cells are reduced, and the bis-fluoroquinolone thiadiazole ureas N-acetyl norfloxacin derivative can be used as anti-tumor active substances to develop anti-tumor drugs withbrand-new structures.
Owner:HENAN UNIVERSITY
Methods for multi-dose synthesis of [f-18]fddnp for clinical settings
InactiveCN109922837ACompensation decaySimplify complex pre-purificationOrganic compound preparationRadiation applicationsFluoroethylEthyl group
A method of manufacturing 2-(1-{6-[(2-[F-18]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)-malononitrile ([F-18]FDDNP) utilizes a semi-automated module that is used to perform fluorination, pre-purification, separation, product extraction, and formulation. The method is able to produce [F-18]FDDNP with high yields and ready for human administration under existing FDA regulations, and without the need for hazardous organic solvents such as dichloromethane (DCM), methanol (MeOH), and tetrahydrofuran (THF). The method also improves the speed with which [F-18]FDDNP can be synthesized with the method being able to generate a final product within about 90 to 100 minutes. This synthesis method is easily adaptable to FDA registered and approved automated synthesis systems.
Owner:RGT UNIV OF CALIFORNIA
Low-temperature electrolyte taking ethyl fluoroacetate as solvent and application of low-temperature electrolyte
PendingCN111952671ALow melting pointImprove oxidation stabilityHybrid capacitor electrolytesSecondary cellsElectrolytic agentFluoroethyl
The invention belongs to the technical field of electrochemistry, and particularly relates to a low-temperature electrolyte taking ethyl fluoroacetate as a solvent and application of the low-temperature electrolyte. According to the low-temperature electrolyte, fluoroethyl acetate and derivatives thereof are used as solvents, lithium salt, sodium salt or quaternary ammonium salt is used as solutes, and the low-temperature electrolyte further comprises a cosolvent and an additive. Compared with the traditional electrolyte, the low-temperature electrolyte disclosed by the invention still shows higher ionic conductivity at lower temperature (minus 80 DEG C). Under the electron withdrawing action of fluorine atoms, fluoroethyl acetate and positive ions have lower desolvation energy, the desolvation process is promoted, and the intercalation and deintercalation of ions at the low temperature are facilitated. When the electrolyte provided by the invention is applied to a lithium ion battery,a sodium ion battery, a supercapacitor and a hybrid supercapacitor, the system shows excellent specific capacity, cycle performance and power performance at low temperature.
Owner:FUDAN UNIV
Preparation and application of bis-fluoroquinolone thiadiazole urea N-methyl lomefloxacin derivatives
ActiveCN109761997AStructural innovationTo achieve the effect of increasing efficiency and reducing toxicityOrganic chemistryAntineoplastic agentsChemical structureSide effect
The invention discloses bis-fluoroquinolone thiadiazole urea N-methyl lomefloxacin derivatives as well as a preparation method and an application thereof. The general chemical structure formula of thederivatives is shown in the formula I as follows in the description. In the formula I, R is ethyl, cyclopropyl, fluoroethyl, an oxazine ring formed with C-8 or a thiazine ring formed with C-8; L is an independent Cl or F atom, 1-piperazinyl, substituted piperazine-1-yl or a nitrogen fused heterocyclic ring; X is CH, N atom or F-substituted C atom (F-C) or a methoxy substituted C atom (CH3O-C). The bis-fluoroquinolone thiadiazole urea N-methyl lomefloxacin derivatives realize organic combination of a bis-fluoroquinolone skeleton and the thiadiazole heterocyclic ring with functional urea pharmacophores, so that transition and superposition of different pharmacophores are realized, anti-tumor activity and selectivity of fluoroquinolone are improved, toxic and side effects on normal cells arereduced, and the derivatives can be taken as an anti-tumor active substance for developing anti-tumor drugs with novel structures.
Owner:HENAN UNIVERSITY
Positron emission tomography (PET) diagnostic radioactive drug and preparation method thereof
ActiveCN101648027BIncreased steric hindranceReduces in vivo degradationIn-vivo radioactive preparationsRadioactive drugFluoroethyl
The invention discloses a positron emission tomography (PET) diagnostic radioactive drug, N-[<18>F]fluoroethyl-N-(1-methylpropyl)-1-(2-chlorophenyl)isoquinoline-3-carbamoyl. The preparation method comprises the following steps: using <18>F<-> and 1,2-dibromoethane to react and prepare labeling intermediate 1-bromo-2-[<18>F]fluoroethane, then using 1-bromo-2-[<18>F]fluoroethane and precursor compound nor-PK11195 to perform alkylation reaction and obtain the finished product. In the method of the invention, [<18>F]ethyl structure is introduced in the molecule of PK11195 so as to increase the space steric effect of molecules, reduce the degradation in vivo of ligand molecules and further increase the signal-to-noise ratio of PET and sensitivity in the detection zone and effectively cover theshortage of the existing PET diagnostic radioactive drug.
Owner:GUANGDONG HUIXUAN PHARMA TECH
Treatment of cardiac dysfunction and heart failure with reduced ejection fraction with compound (r)-4-(1-((3-(difluoromethyl)-1-methyl-1h-pyrazol-4-yl) sulfonyl)-1-fluoroethyl)-n-(isoxazol-3-yl) piperidine-1-carboxamide
Provided herein are methods, uses, and compositions for treating systolic dysfunction, such as heart failure with reduced ejection fraction.
Owner:MYOKARDIA
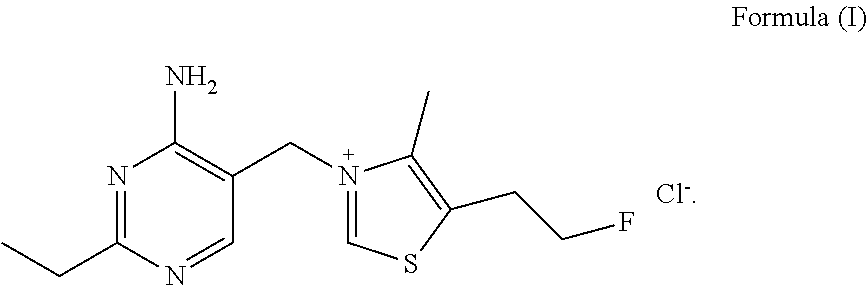
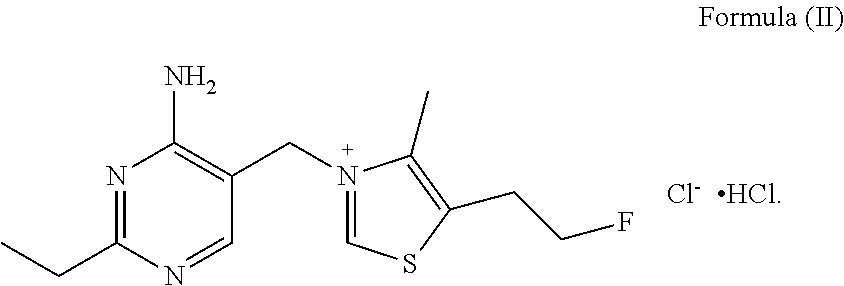
Fluoroethyl thiamine or salts thereof and application thereof in preparation of anticoccidial drugs
ActiveUS9096581B2Remarkable activityOrganic chemistryAnimal feeding stuffAnticoccidial AgentsThiamine
The present invention discloses a fluoroethyl thiamine or salts thereof and application thereof in preparation of anticoccidial drugs. The structural formula of the fluoroethyl thiamine or salts thereof is shown as Formula (I). The fluoroethyl thiamine or salts thereof of the present invention have a remarkable anticoccidial effect, particularly on some coccidia which had resistance to other anticoccidial drugs, therefore the fluoroethyl thiamine or salts thereof of the present invention can be applied to preparation of anticoccidial drugs. Thus, the present invention provides conditions for development of new anticoccidial drugs.
Owner:GUANGZHOU INSIGHER BIOTECHNOLOGY CO LTD
7-{(3s,4s)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidin-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methyl Oxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal
ActiveCN103930414BImprove solubilityGood storage stabilityAntibacterial agentsOrganic active ingredientsQuinolineFluoroethyl
The object of the present invention is to provide hydrochloride crystals, hydrochloride hydrate crystals and methanesulfonate crystals of the compound represented by formula (1). Compared with the free crystals of compound (1), these crystals are less prone to be decomposed by the action of light, and also have higher storage stability and higher water solubility.
Owner:KYORIN PHARMA CO LTD
Silane-modified fluoroethyl ester polymer used as lithium battery binder and preparation method thereof
ActiveCN111925471BSimple structureFunction increaseCell electrodesSecondary cellsRotary evaporatorSilanes
The invention discloses a preparation method of a silane-modified fluoroethyl ester polymer used as a lithium battery binder, which comprises the following steps: 1) mixing perfluorovinyl ester compounds, silane compounds, 80%- Add 90% of the solvent into the container and stir, heat up to 60-80°C, dissolve the initiator in the remaining solvent and add it dropwise to the reaction solution. After the addition is complete, keep warm and continue the reaction for 10-12h; The solution was transferred to a rotary evaporator, the solvent was evaporated, the precipitate was washed with n-hexane, and the precipitate was dried to obtain the silane-modified fluoroethyl ester polymer. The fluorine atoms in the polymer prepared by the invention have a good binding force with lithium ions, which accelerates the transfer of lithium ions and improves the Coulombic efficiency of the battery; the silane structure enhances the adhesion between the polymer and the inorganic active silicon particles, and improves the battery life The cycle stability of the prepared polymers has excellent initial Coulombic efficiency and electrochemical stability.
Owner:HUBEI UNIV
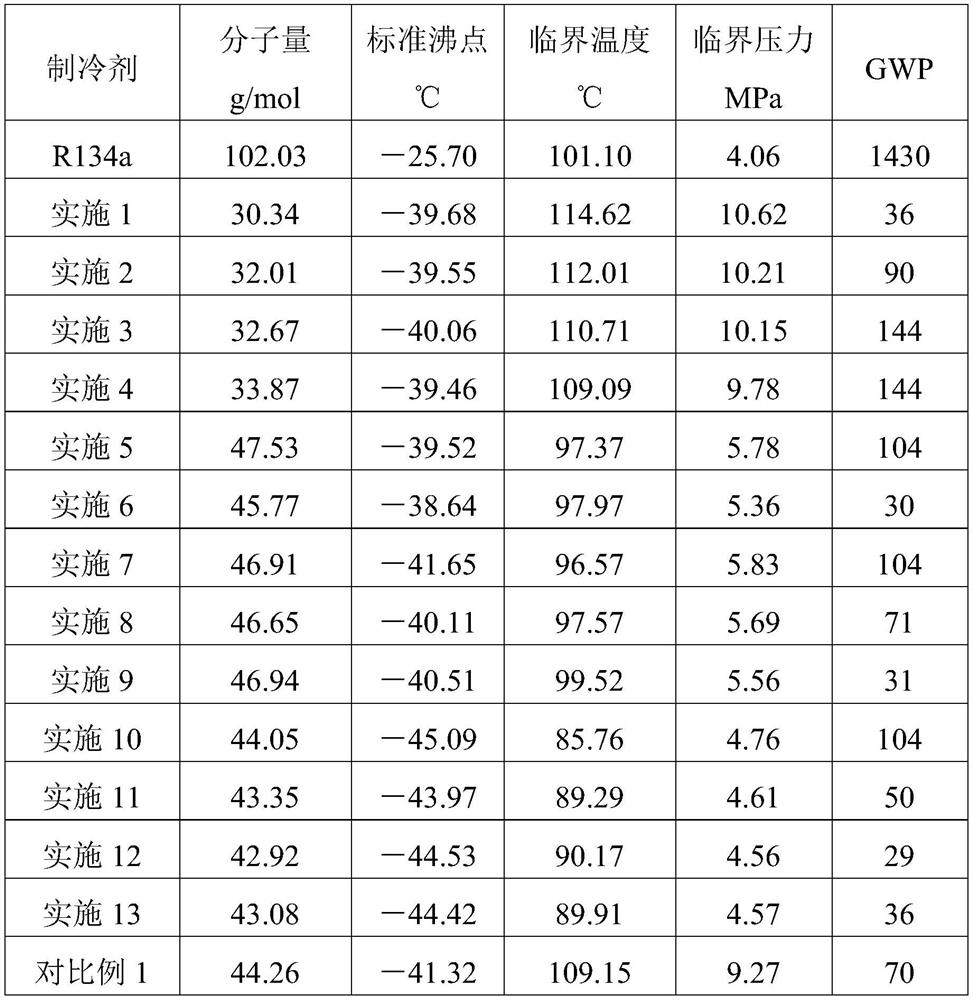
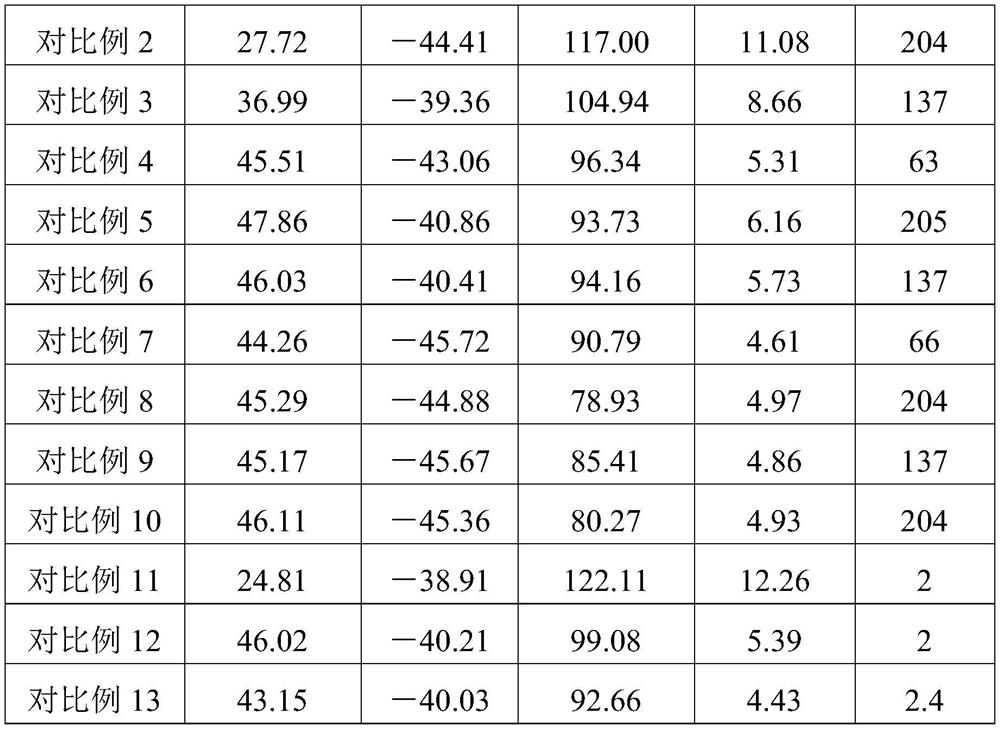
A kind of mixed refrigerant and its preparation method and application, automobile air-conditioning system
ActiveCN112126410BLow GWP valueExcellent GWP valueAir-treating devicesVehicle heating/cooling devicesAlkaneEngineering
The invention discloses a mixed refrigerant, its preparation method and application, and an automobile air-conditioning system, relates to the technical field of air-conditioning, and solves the technical problems of high GWP value and low COP of R134a refrigerant in the prior art. The mixed refrigerant of the present invention includes a first component, a second component and a third component, wherein the first component is propane, the second component is difluoromethane, and the third component is ammonia, fluoroethyl One of alkane and propylene. The mixed refrigerant of the present invention has a COP superior to that of R134a refrigerant while having a low GWP value. Specifically, the GWP value of the mixed refrigerant of the present invention is lower than 150, which meets the requirements of environmental protection regulations in various regions of the world. Under proper proportioning, the thermodynamic performance is not only better than that of R134a refrigerant, but also the glide temperature of the mixed refrigerant of the present invention is less than 1°C, the adverse effects of temperature glide can be ruled out. That is, the mixed refrigerant of the present invention has good environmental protection performance and thermal performance, which is better than that of R134a refrigerant.
Owner:GREE ELECTRIC APPLIANCES INC
Polymorphic forms of (r)-4-(1-((3-(difluoromethyl)-1-methyl-1h-pyrazol-4-yl)sulfonyl)-1-fluoroethyl)-n-(isoxazol-3-yl)piperidine-1-carboxamide
The present invention provides novel polymorphs of (R)-4-(1-(3-(difluoromethyl)-1-methyl-1H-pyrazol-4-yl)sulfonyl)-1-fluoroethyl)-N-(isoxazol-3-yl)piperidine-1-carboxamide (I-491) that are useful for the treatment of cardiac disorders including systolic dysfunction, dilated cardiomyopathy (DCM), heart failure with reserved ejection fraction (HFrEF), and conditions associated with left and / or right ventricular systolic dysfunction or systolic reserve. The synthesis and characterization of the polymorphs is described, as well as methods for treating systolic dysfunction, DCM, HFrEF, and other forms of heart disease.
Owner:MYOKARDIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Convenient method for the preparation of new precursor of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine) Convenient method for the preparation of new precursor of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine)](https://images-eureka.patsnap.com/patent_img/bd75da01-a433-4227-b3b3-e2a24bcf0b79/US07138540-20061121-C00001.png)
![Convenient method for the preparation of new precursor of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine) Convenient method for the preparation of new precursor of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine)](https://images-eureka.patsnap.com/patent_img/bd75da01-a433-4227-b3b3-e2a24bcf0b79/US07138540-20061121-C00002.png)
![Convenient method for the preparation of new precursor of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine) Convenient method for the preparation of new precursor of no-carrier-added O-(2-[18F]fluoroethyl)-L-Tyrosine)](https://images-eureka.patsnap.com/patent_img/bd75da01-a433-4227-b3b3-e2a24bcf0b79/US07138540-20061121-C00003.png)
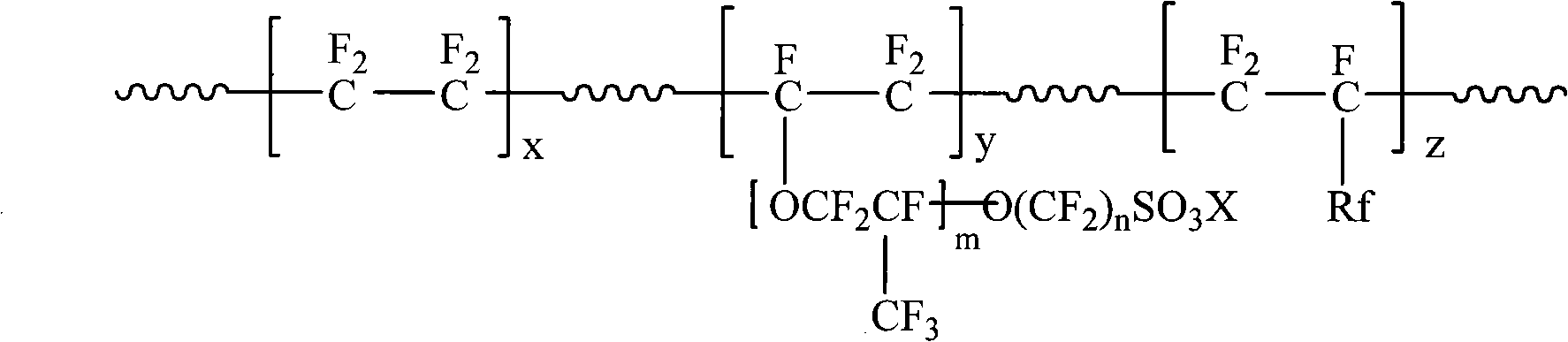
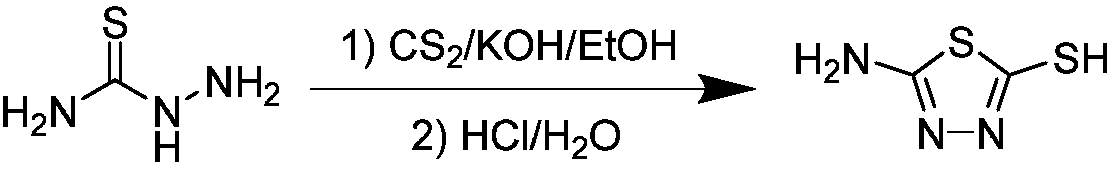
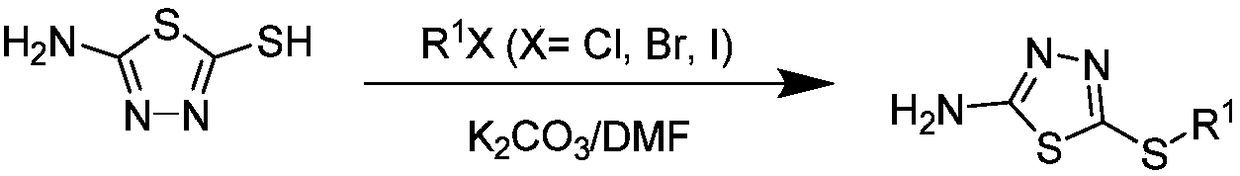
![7-{(3S,4S)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidine-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal 7-{(3S,4S)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidine-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal](https://images-eureka.patsnap.com/patent_img/0f00bab4-0d2c-48af-8b00-e457d73803aa/HDA0000502915930000011.PNG)
![7-{(3S,4S)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidine-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal 7-{(3S,4S)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidine-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal](https://images-eureka.patsnap.com/patent_img/0f00bab4-0d2c-48af-8b00-e457d73803aa/HDA0000502915930000012.PNG)
![7-{(3S,4S)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidine-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal 7-{(3S,4S)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidine-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal](https://images-eureka.patsnap.com/patent_img/0f00bab4-0d2c-48af-8b00-e457d73803aa/HDA0000502915930000013.PNG)
![Convenient method for the preparation of no-carrier-added O-(2-[18F]fluoroethyl)-L-tyrosine) Convenient method for the preparation of no-carrier-added O-(2-[18F]fluoroethyl)-L-tyrosine)](https://images-eureka.patsnap.com/patent_img/570a6953-5196-4ff4-96cd-f843811ba511/US07132563-20061107-D00001.png)
![Convenient method for the preparation of no-carrier-added O-(2-[18F]fluoroethyl)-L-tyrosine) Convenient method for the preparation of no-carrier-added O-(2-[18F]fluoroethyl)-L-tyrosine)](https://images-eureka.patsnap.com/patent_img/570a6953-5196-4ff4-96cd-f843811ba511/US07132563-20061107-D00002.png)
![Convenient method for the preparation of no-carrier-added O-(2-[18F]fluoroethyl)-L-tyrosine) Convenient method for the preparation of no-carrier-added O-(2-[18F]fluoroethyl)-L-tyrosine)](https://images-eureka.patsnap.com/patent_img/570a6953-5196-4ff4-96cd-f843811ba511/US07132563-20061107-D00003.png)
![Methods for multi-dose synthesis of [f-18]fddnp for clinical settings Methods for multi-dose synthesis of [f-18]fddnp for clinical settings](https://images-eureka.patsnap.com/patent_img/f6fd8072-6383-45d8-8a46-f5429c3effb1/HDA0002051352430000011.png)
![Methods for multi-dose synthesis of [f-18]fddnp for clinical settings Methods for multi-dose synthesis of [f-18]fddnp for clinical settings](https://images-eureka.patsnap.com/patent_img/f6fd8072-6383-45d8-8a46-f5429c3effb1/HDA0002051352430000021.png)
![Methods for multi-dose synthesis of [f-18]fddnp for clinical settings Methods for multi-dose synthesis of [f-18]fddnp for clinical settings](https://images-eureka.patsnap.com/patent_img/f6fd8072-6383-45d8-8a46-f5429c3effb1/HDA0002051352430000031.png)
![7-{(3s,4s)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidin-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methyl Oxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal 7-{(3s,4s)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidin-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methyl Oxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal](https://images-eureka.patsnap.com/patent_img/09f7d136-8159-4f84-bf3f-6dedd8ab4d57/HDA0000502915930000011.PNG)
![7-{(3s,4s)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidin-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methyl Oxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal 7-{(3s,4s)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidin-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methyl Oxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal](https://images-eureka.patsnap.com/patent_img/09f7d136-8159-4f84-bf3f-6dedd8ab4d57/HDA0000502915930000012.PNG)
![7-{(3s,4s)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidin-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methyl Oxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal 7-{(3s,4s)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidin-1-yl}-6-fluoro-1-(2-fluoroethyl)-8-methyl Oxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid crystal](https://images-eureka.patsnap.com/patent_img/09f7d136-8159-4f84-bf3f-6dedd8ab4d57/HDA0000502915930000013.PNG)