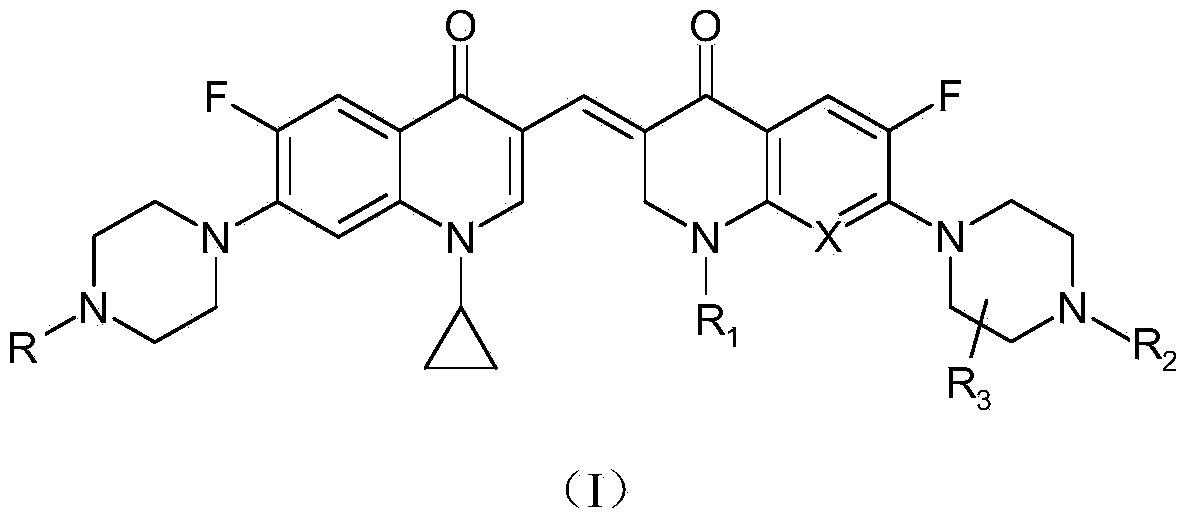
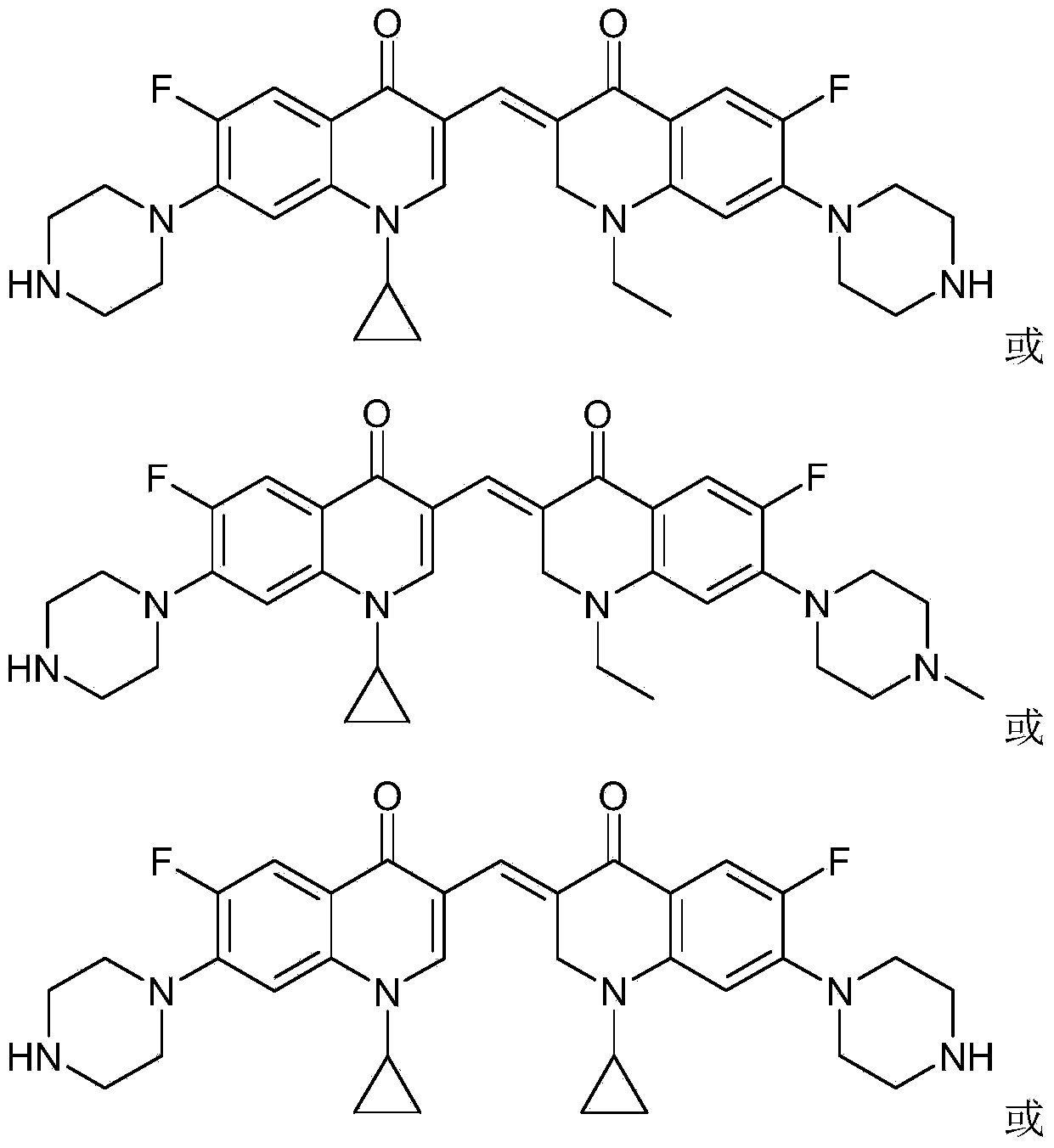
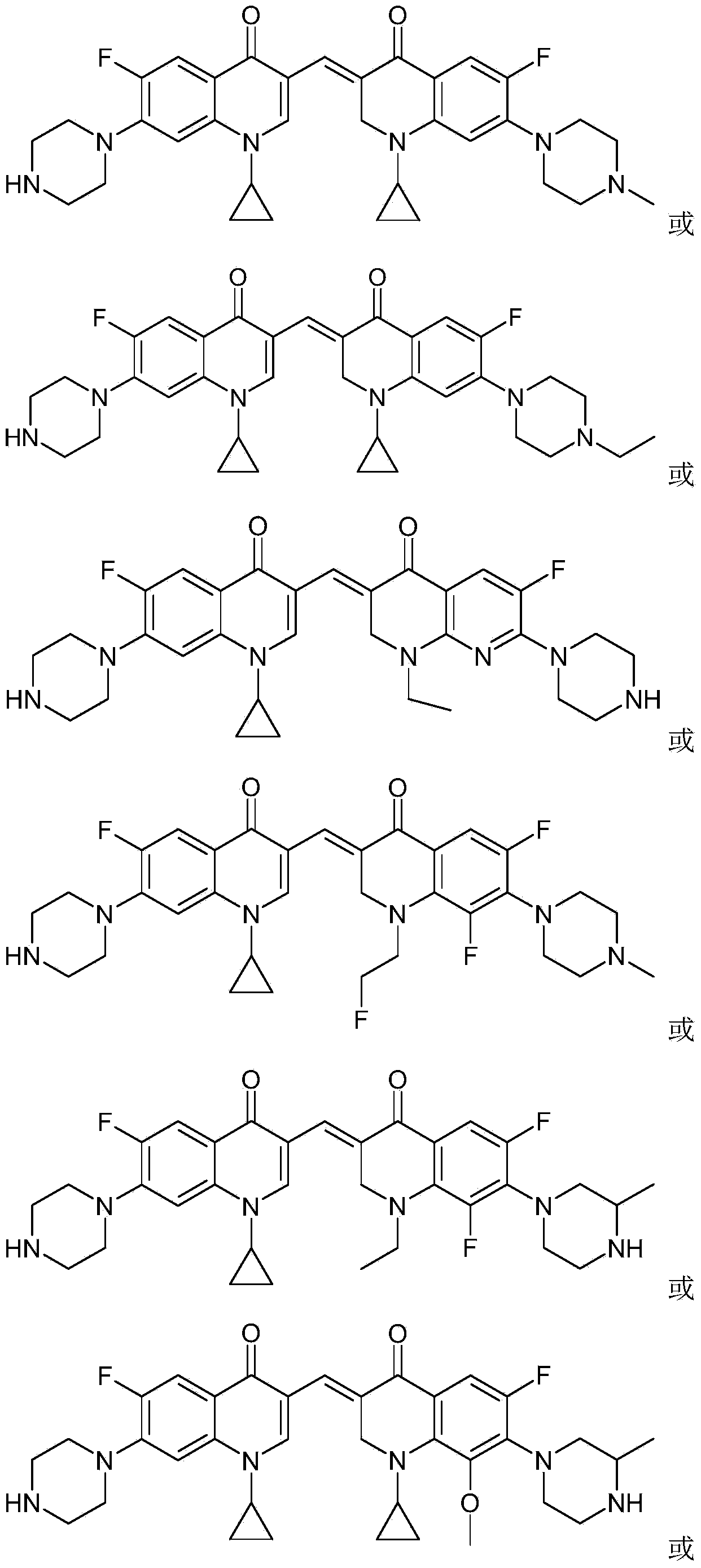
3,3'-methylene-bisfluoroquinolone derivative containing cyclopropylquinoline ring as well as preparation method and application of 3,3'-methylene-bisfluoroquinolone derivative
A technology of cyclopropaquinoline ring and difluoroquinolone, which is applied in the field of difluoroquinolone derivative compounds, can solve the problems of high toxicity of antitumor drugs, poor patient tolerance, low cure rate of tumor diseases, etc., and achieves increased antitumor activity, The effect of reducing toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The 3,3'-methylene-bisfluoroquinolone derivative containing cyclopropaquinoline ring in this embodiment is 1-cyclopropyl-6-fluoro-7-piperazin-1-yl-3-[1- Ethyl-6-fluoro-7-piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]quinolin-4(1H)-one, which The chemical structural formula is:
[0044]
[0045] That is, R in the formula (I) is a hydrogen atom, and R 1 is ethyl, R 2 and R 3 are hydrogen atoms, and X is a hydrocarbon group.
[0046] The preparation method of the 3,3'-methylene-bisfluoroquinolone derivative containing cyclopropaquinoline ring in this example is as follows: take 0.50 g (1.6 mmol) of 1-cyclopropyl-6-fluoro-7- Piperazin-1-yl-quinolin-4(1H)-one-3-carbaldehyde and 0.44g (1.6mmol) of 1-ethyl-6-fluoro-7-piperazin-1-yl-2,3- Dihydro-quinolin-4(1H)-one was dissolved in 20ml of absolute ethanol, 0.2ml of piperidine was added dropwise, and after reflux reaction for 24h, it was allowed to stand overnight, and the resulting solid was collected by...
Embodiment 2
[0048] The 3,3'-methylene-bisfluoroquinolone derivative containing cyclopropaquinoline ring in this embodiment is 1-cyclopropyl-6-fluoro-7-piperazin-1-yl-3-[1- Ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]quinoline-4( 1H)-ketone, its chemical structural formula is:
[0049]
[0050] That is, R in formula I is a hydrogen atom, R 1 is ethyl, R 2 is methyl, R 3 is a hydrogen atom, and X is a hydrocarbon group.
[0051] The preparation method of the 3,3'-methylene-bisfluoroquinolone derivative containing cyclopropaquinoline ring in this example is as follows: take 0.50 g (1.6 mmol) of 1-cyclopropyl-6-fluoro-7- Piperazin-1-yl-quinolin-4(1H)-one-3-carbaldehyde and 0.47g (1.6mmol) of 1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl )-2,3-dihydro-quinolin-4(1H)-one, dissolved in 20ml of absolute ethanol, added dropwise 0.2ml of piperidine, after reflux for 24h, left overnight, filtered the resulting solid, and washed with DMF - Recrystall...
Embodiment 3
[0053] The 3,3'-methylene-bisfluoroquinolone derivative containing cyclopropaquinoline ring in this embodiment is 1-cyclopropyl-6-fluoro-7-piperazin-1-yl-3-[1- Cyclopropyl-6-fluoro-7-piperazin-1-yl-2,3-dihydro-quinolin-4(1H)-one-3-ylidenemethyl]quinolin-4(1H)-one, Its chemical structural formula is:
[0054]
[0055] That is, R in formula I is a hydrogen atom, R 1 is cyclopropyl, R 2 and R 3 is a hydrogen atom, and X is a hydrocarbon group.
[0056] The preparation method of the 3,3'-methylene-bisfluoroquinolone derivative containing cyclopropaquinoline ring in this embodiment is as follows: take 0.50g (1.6mmol) 1-cyclopropyl-6-fluoro-7-piper Azin-1-yl-quinolin-4(1H)-one-3-carbaldehyde and 0.46g (1.6mmol) of 1-cyclopropyl-6-fluoro-7-piperazin-1-yl-2,3- Dihydro-quinolin-4(1H)-one was dissolved in 20ml of absolute ethanol, 0.2ml of piperidine was added dropwise, and after reflux reaction for 24h, it was allowed to stand overnight, and the resulting solid was collected by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com