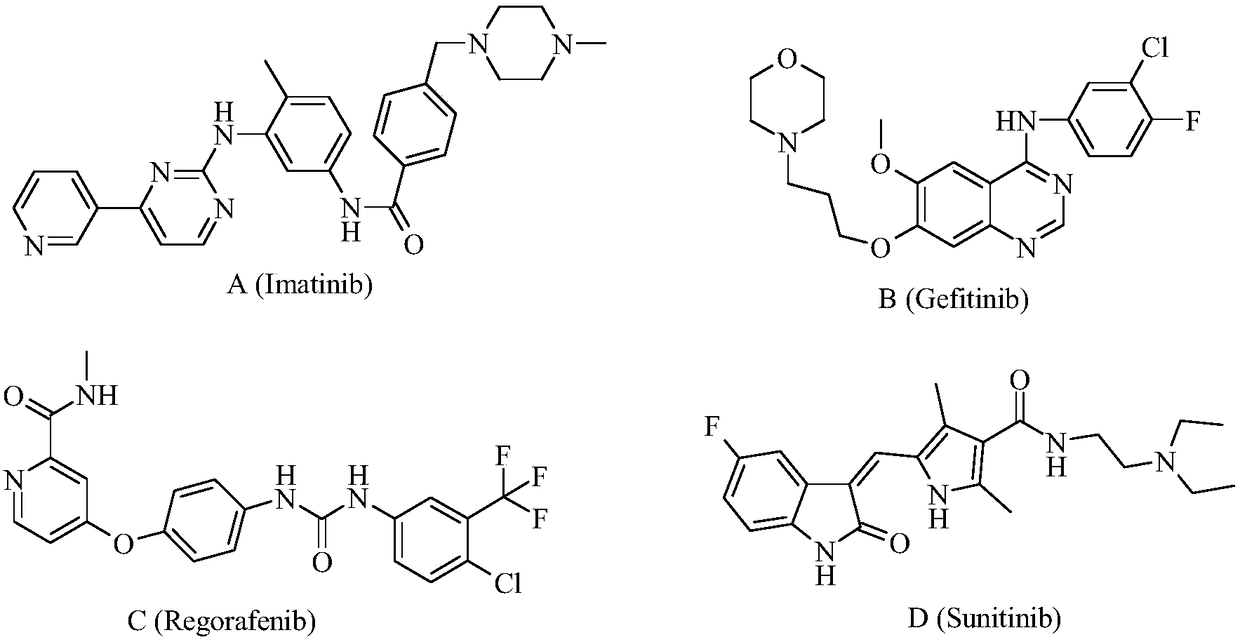
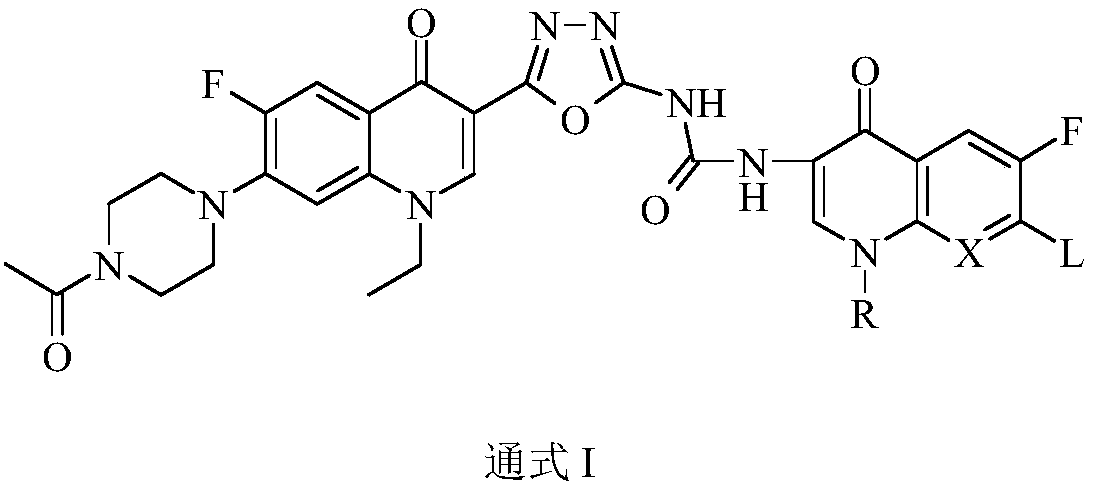
Bi-fluoroquinolone oxadiazole ureas N-acetyl norfloxacin derivative and preparation method and application thereof
A technology of fluoroquinolone oxadiazuron and acetyl norfloxacin, which is applied in the design of bis-fluoroquinolone oxadiazuron derivatives of N-acetyl norfloxacin, the preparation of such derivatives, and antineoplastic drugs In the field of application, to achieve the effect of innovative structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
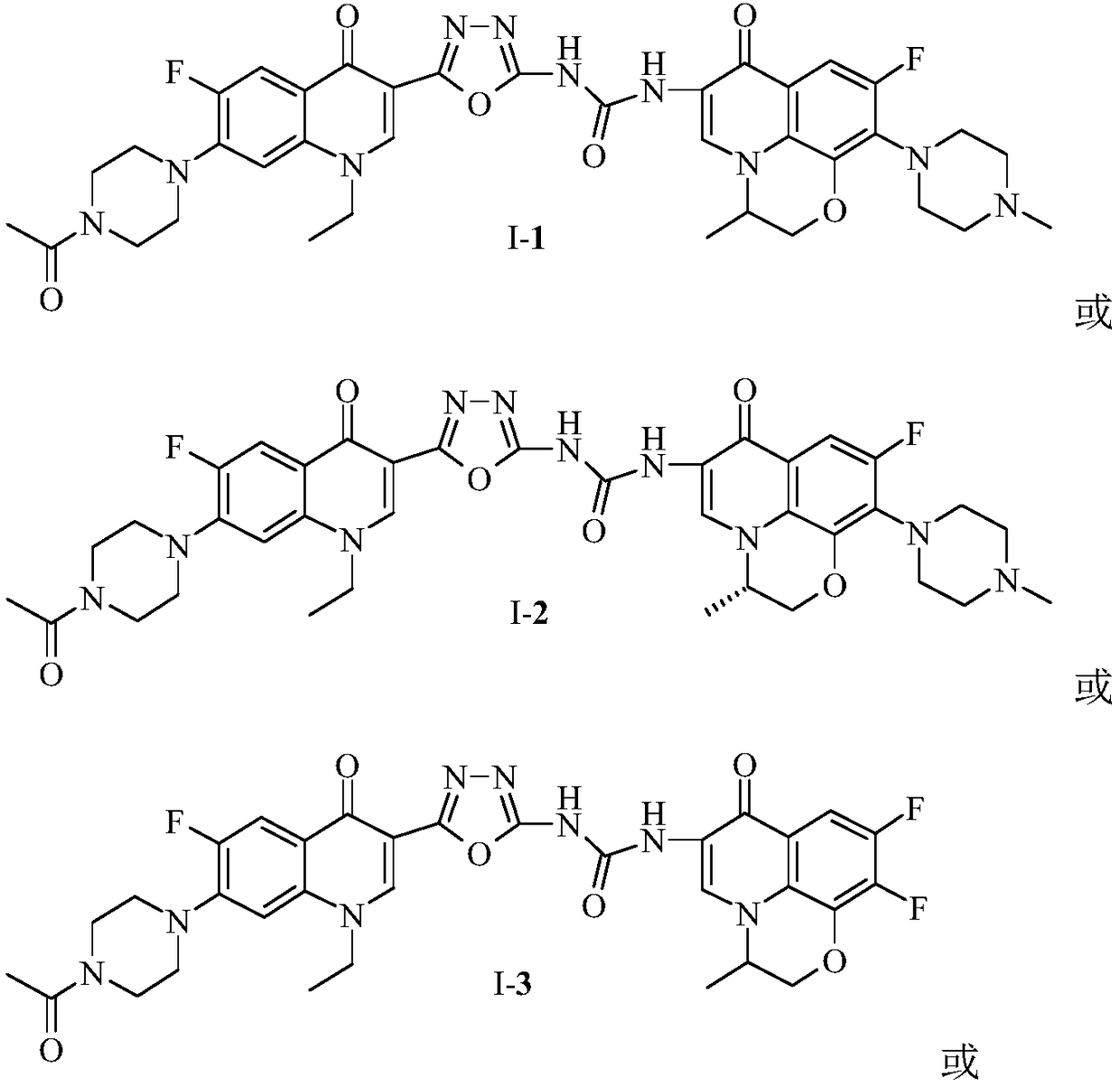
[0054] 1-{2-[1-Ethyl-6-fluoro-7-(4-acetylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-1,3,4 -Oxadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)-quinoline-4 (1H)-ketone-3-yl]-urea (I-1), its chemical structural formula is:
[0055]
[0056] The preparation method of the bis-fluoroquinolone oxadiazuron of the present embodiment is: get ofloxacin hydroxamic acid (1 ") 1.0g (2.7mmol) and suspend in 25mL acetonitrile, add carbonyldiimidazole (CDI) 0.67g (4.1mmol), stirring at room temperature until the material is dissolved. Then add N-acetyl norfloxacin C-3 oxadiazole amide intermediate II 1.08g (2.7mmol), stir in a water bath at 55-60°C for 16 hours. Leave overnight and filter The resulting solid was collected and washed with acetonitrile. The crude product was recrystallized from a mixed solvent of DMF-ethanol to obtain a light yellow crystal (I-1), with a yield of 61%, m.p.225-227°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 11.57 (brs, 1H, NH), 9.48 (s, 1H, NH), ...
Embodiment 2
[0058] (S)-1-{2-[1-Ethyl-6-fluoro-7-(4-acetylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-1 ,3,4-oxadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)- Quinoline-4(1H)-ketone-3-yl]-urea (I-1), its chemical structural formula is:
[0059]
[0060] The preparation method of the bis-fluoroquinolone oxadiazuron of the present embodiment is: take levofloxacin hydroxamic acid (2″) 1.0g (2.7mmol) and suspend in 25mL acetonitrile, add carbonyldiimidazole (CDI) 0.60g (3.7mmol ), stirred at room temperature until the material was dissolved. Then 1.08 g (2.7 mmol) of N-acetyl norfloxacin C-3 oxadiazolamide intermediate II was added, stirred in a water bath at 55-60° C. for 10 hours. Placed overnight, filtered and collected The solid was washed with acetonitrile. The crude product was recrystallized from ethanol to obtain a light yellow crystal (I-2), with a yield of 50%, m.p.213-215°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 11.57(brs, 1H, NH), 9.46(s, 1H, NH), 9.20, 9.03(2s...
Embodiment 3
[0062] 1-{2-[1-Ethyl-6-fluoro-7-(4-acetylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-1,3,4 -Oxadiazol-5-yl}-3-[6,7-difluoro-1,8-(1,3-oxopropyl)-quinolin-4(1H)-one-3-yl]-urea (I-3), its chemical structural formula is:
[0063]
[0064] The preparation method of the bis-fluoroquinolone oxadiazuron of the present embodiment is: take 1.0 g (3.4 mmol) of oxyfluorocarboxylic acid hydroxamic acid (3″) and suspend it in 25 mL of acetonitrile, add 0.82 g of carbonyldiimidazole (CDI) (5.1mmol), stir at room temperature until the material dissolves. Then add N-acetyl norfloxacin C-3 oxadiazolamide intermediate II 1.36g (3.4mmol), stir in a water bath at 55-60°C for 24 hours. Leave overnight and filter The resulting solid was collected and washed with acetonitrile. The crude product was recrystallized from a DMF-ethanol mixed solvent to obtain a light yellow crystal (I-3), with a yield of 65%, m.p.222-224°C. 1 H NMR (400MHz, DMSO-d6 )δ:11.56(brs,1H,NH),9.47(s,1H,NH),9.16,8.92(2s,2H,2×2′...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com