Continuous synthesis method for 2-fluoroethyl malonate compound
The technology of a fluoromalonate diester and a synthesis method, which is applied in the field of medicine and chemical industry, can solve the problems of troublesome treatment, low production efficiency, and many three wastes, etc., and achieves the effects of reducing the high temperature dangerous area, avoiding the decomposition of raw materials, and reducing the safety risk.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
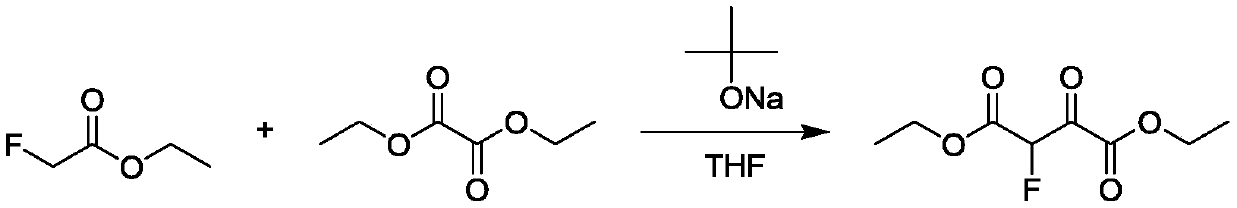
[0022] According to a typical embodiment of the present invention, a continuous synthesis method of 2-fluoromalonate diester compounds is provided. The continuous synthesis method comprises the following steps: As a raw material, a continuous decarbonylation reaction is carried out in a continuous reaction device to obtain 2-fluoromalonate diester compounds Wherein, R and R' represent linear or branched alkyl, substituted or unsubstituted aryl, substituted or unsubstituted heterocyclic or cyclic alkyl, respectively, and R and R' can be the same or different.
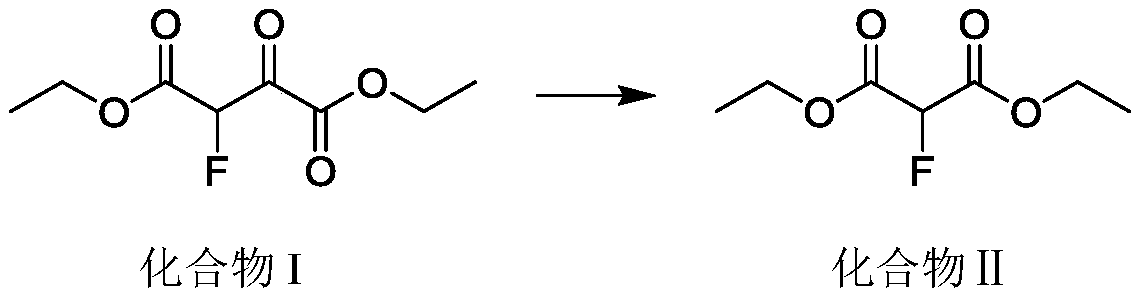
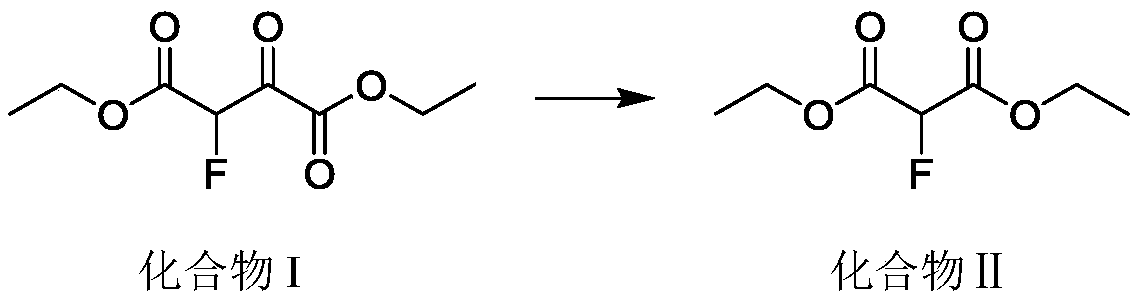
[0023] The invention adopts continuous reaction equipment to realize the high-temperature carbonyl elimination reaction of 2-fluoro-3-oxosuccinic acid diester. Compared with the traditional kettle reaction, the amount of materials participating in the reaction per unit time is greatly reduced, the high temperature danger area is reduced, and the safety risk is greatly reduced. The continuous reactor can instantly heat...
Embodiment 1
[0037]
[0038] Compound I was synthesized with reference to Chinese patent (CN 102531897). In a 1000L reactor, 614.0kg of tetrahydrofuran (1kg / 6L) was added at one time, and 156.3kg of sodium tert-butoxide (1.5eq) and 136.5kg of diethyl oxalate were added in batches. (1.3eq), temperature control 25±2°C, dropwise add 115kg main raw material ethyl fluoroacetate (1.0eq) to the system, heat preservation reaction, after the reaction is complete, carry out post-treatment with temperature control 25±2°C, use 690kg 10 % hydrochloric acid solution (1kg / 6kg) to adjust the pH=2, then extract and wash, add 17.3kg of silica gel (1g / 0.15g) to remove tar, and finally dry, press filter and concentrate to obtain the product 2-fluoro- 217.2 kg of diethyl 3-oxosuccinate, yield 72.9%, external standard (Wt%): 75%, liquid chromatography purity (HPLC): 91.2%.
Embodiment 2
[0040]
[0041] The compound I dosage is 1.0kg (3.64mol), and the compound I is pumped into the continuous coil reactor (volume 15mL) at a rate of 1.2g / min, the retention time is: 15min, the temperature is controlled at 350°C, and the reaction is continuous The backup pressure at the outlet of the device is 2.5-3.0MPa, the outlet sampling GC analysis, the remaining raw material is 0.5-3.0%, the outflow system is rectified to obtain the product 2-fluoromalonate (II) 595.0g, gas phase purity: 98.1% , the yield was 90%, and the NMR identification was correct.
[0042] The parameters of rectification are: column temperature 90°C, kettle liquid temperature 106.9-142°C, vacuum degree 9mmHg, tower top temperature: 84-78.4, reflux ratio: 12 / 4;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com