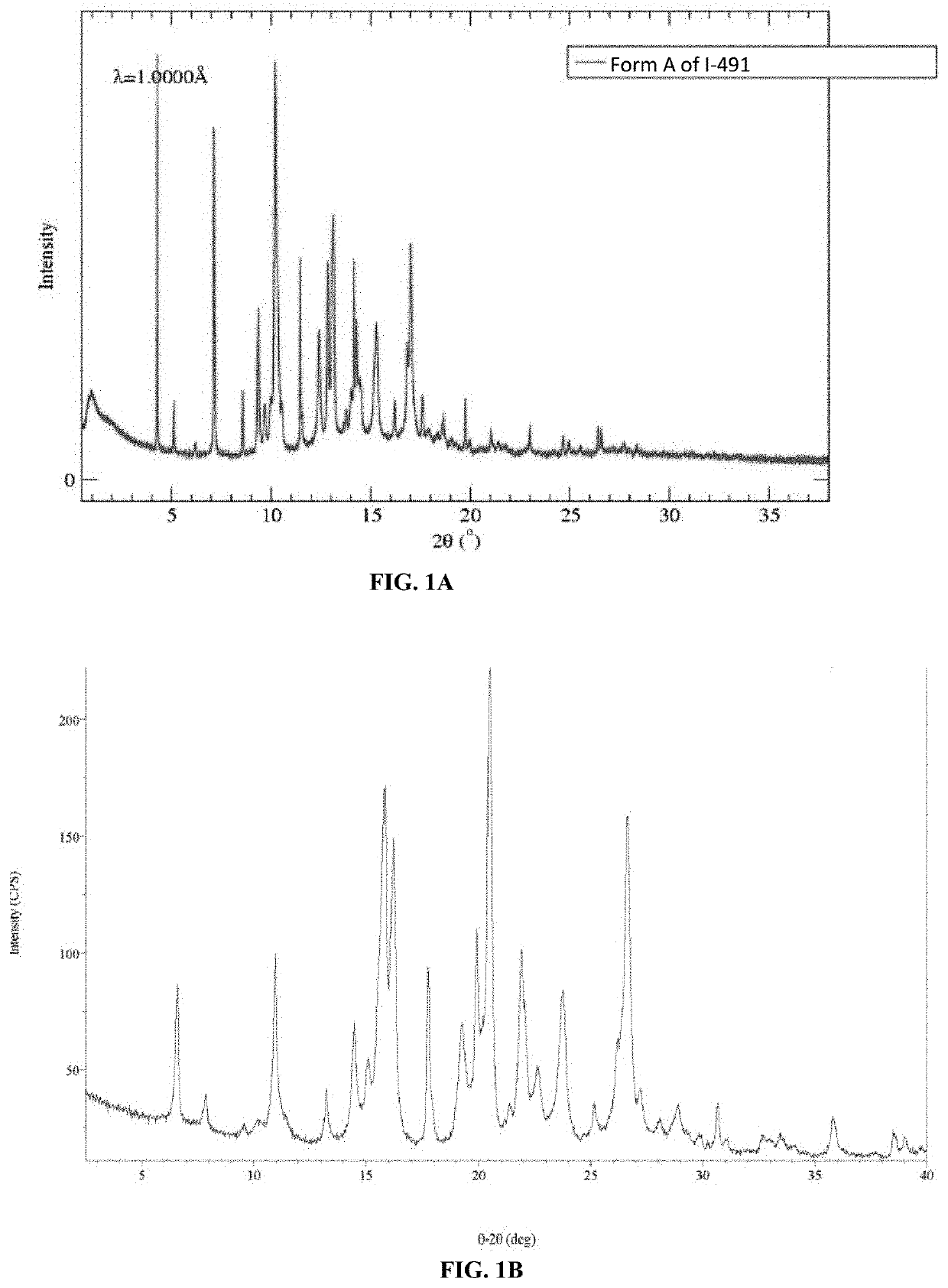
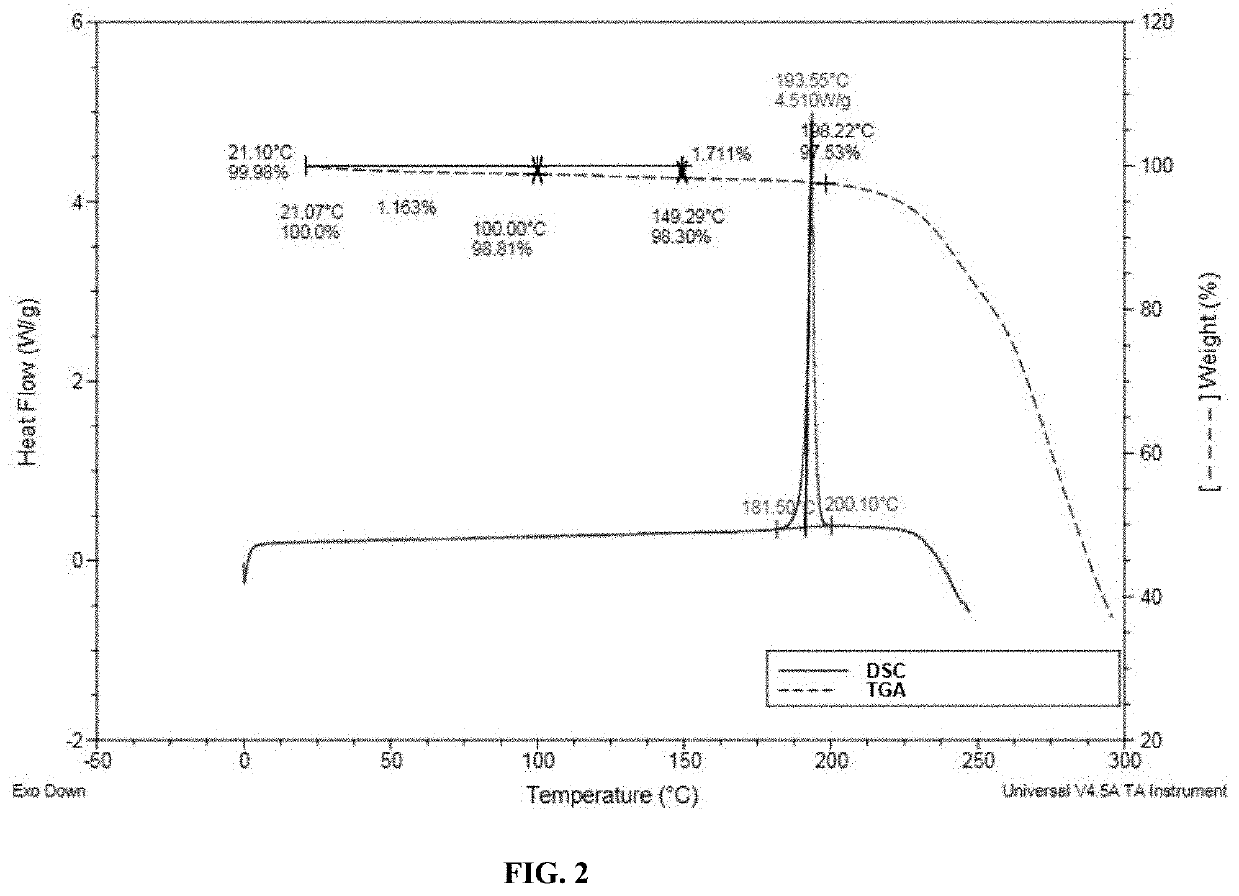
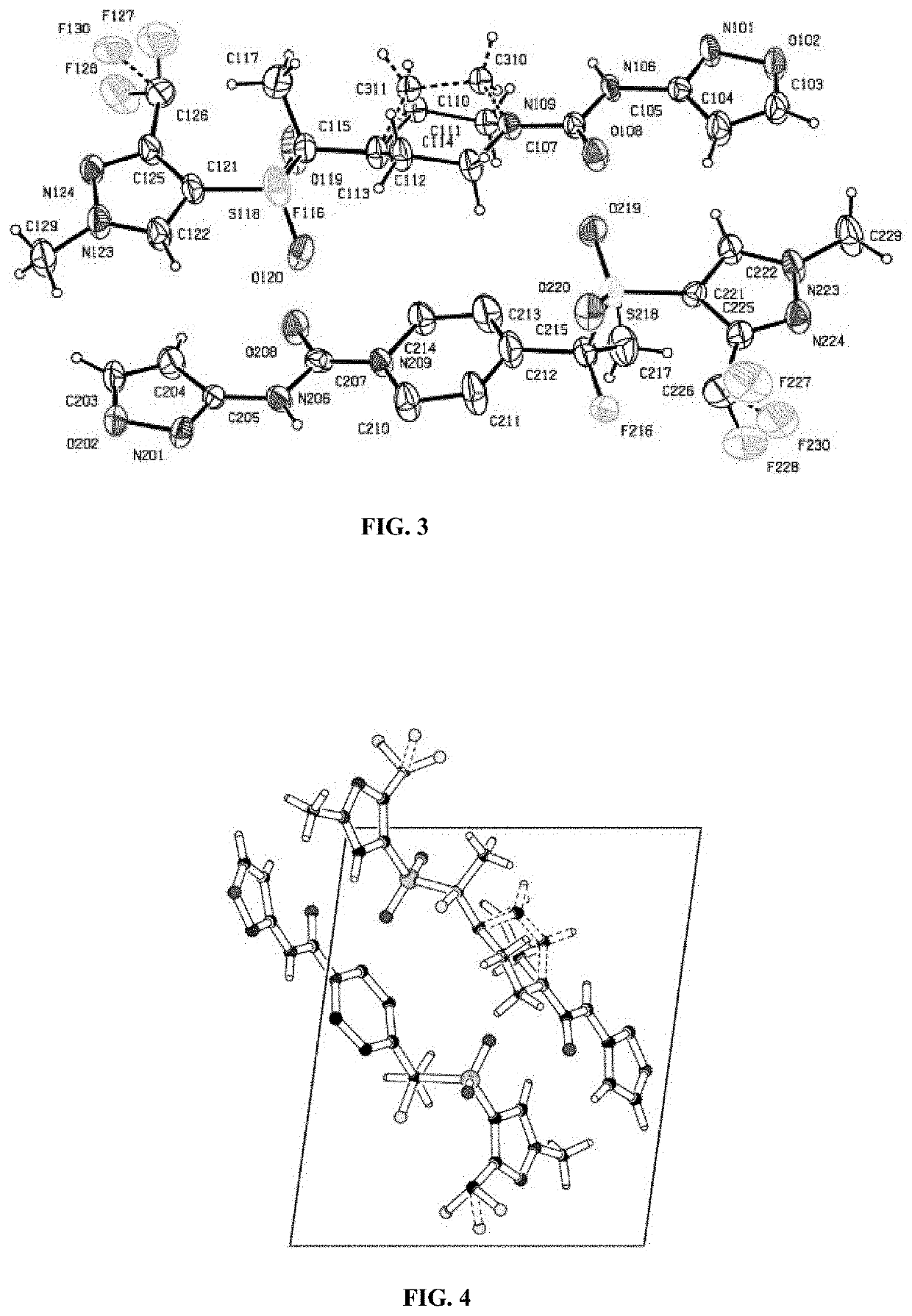
Polymorphic forms of (r)-4-(1-((3-(difluoromethyl)-1-methyl-1h-pyrazol-4-yl)sulfonyl)-1-fluoroethyl)-n-(isoxazol-3-yl)piperidine-1-carboxamide
a polymorphic form and piperidine technology, applied in the field of polymorphic forms of (r)4(1(3(difluoromethyl)1methyl1hpyrazol4yl) sulfonyl)1fluoroethyl)n(isoxazol3yl) piperidine1carboxamide, can solve the problems of more complex and heterogeneous pathophysiology, difficulty in pumping enough blood to support other organs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of (R)-4-(1-((3-(Difluoromethyl)-1-methyl-1H-pyrazol-4-yl)sulfonyl)-1-fluoroethyl)-N-(isoxazol-3-yl)piperidine-1-carboxamide (I-491)
[0137]I-491 was synthesized as described in U.S. Pat. No. 9,925,177.
example 2
Evaporation Experiments
[0138]Solutions of I-491 were prepared in various solvents at room temperature. Once the mixtures reached complete dissolution, as judged by visual observation, solutions were allowed to evaporate to dryness from an open vial at room temperature. The solids were analyzed by XRPD.
TABLE 1SolventPolymorph ObtainedAcetonitrileForm A + εDAcetoneForm A + acetone solvateMethyl Ethyl KetoneForm A + εDDichloromethaneForm A + εD
example 3
Slurry Experiments
[0139]The selected solvent was pre-saturated by slurring with I-491 at the selected temperature. A small amount (20 mg / mL) of I-491 was then added and the suspensions were slurried for two weeks at the indicated temperature. The solids were collected by vacuum filtration and analyzed by XRPD and TGA. The results obtained are reported in Table 2 and Table 3.
[0140]In a first set of experiments (Table 2), which were performed utilizing various amounts of water, a non-hydrated form designated as Form B was isolated. In a second set of experiments (Table 3), Form B was isolated by slurring in various solvents without water and at room temperature. At 50° C., a mixture of Forms A and / or B plus Form C was obtained.
TABLE 2SolventPercent WaterTemperaturePolymorph ObtainedEthanol / Water 25%RTForm BEthanol / Water 45%RTForm BEthanol / Water 75%RTForm BMethanol / Water 25%RTForm BMethanol / Water 45%RTForm BMethanol / Water 75%RTForm BWater100%RTForm B
TABLE 3SolventTemperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com