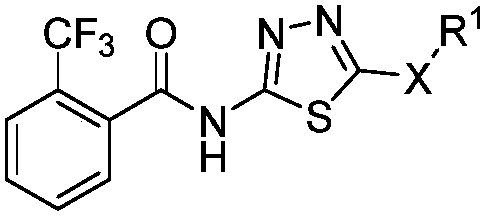
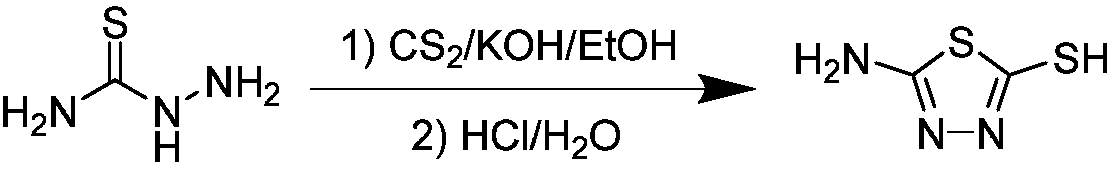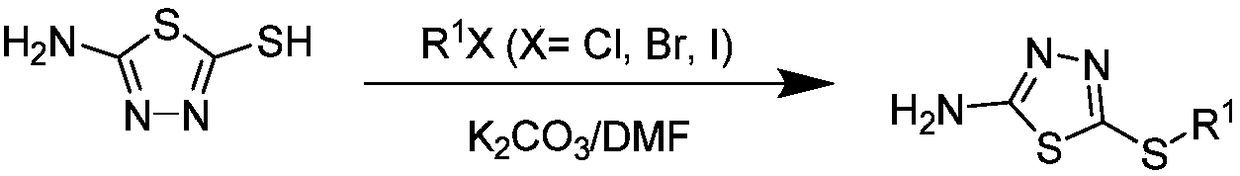1,3,4-thiadiazole thioether (sulfone)-containing 2-(trifluoromethyl)benzamide derivative and preparation and application thereof
A technology of thiadiazole sulfide and benzamide, which is applied to 2-(trifluoromethyl)benzamide derivatives containing 1,3,4-thiadiazole sulfide (sulfone), its preparation and Application field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: N-(2-((3-chloro-5-(trimethyl)pyridin-2-yl)sulfur)-1,3,4-thiadiazol-2-yl)-5-(tri The synthetic (compound number is A1) of fluoromethyl) benzamide comprises the following steps:
[0058] (1) Put thiosemicarbazide (10.0mmol), potassium hydroxide (15.0mmol) and absolute ethanol (60mL) as the solvent into a 100mL three-neck flask, stir continuously at room temperature until the solid dissolves and slowly add carbon disulfide ( 15.0mmol), and then heated to reflux for 8-10h. After the reaction was completed, the ethanol removed under reduced pressure was added to ice water, and the pH was adjusted to 3-4 with 5% hydrochloric acid, and then the solid was collected by filtration and recrystallized with absolute ethanol to obtain intermediate 2-amino-5-mercapto-1,3,4-thiadiazole;
[0059] (2) Put 2-amino-5-mercapto-1,3,4-oxadiazole (4.0mmol), potassium carbonate (6.0mmol) and DMF (2mL) as solvent into a 100mL three-necked flask, and keep stirring until After the sol...
Embodiment 2
[0061] Example 2: Synthesis of N-(5-(methylthio)-1,3,4-thiadiazol-2-yl)-2-(trifluoromethyl)benzamide (compound number is A2), Include the following steps:
[0062] (1) with the (1) step of embodiment 1;
[0063] (2) Put 2-amino-5-mercapto-1,3,4-oxadiazole (4.0mmol), potassium carbonate (6.0mmol) and DMF (2mL) as solvent into a 100mL three-necked flask, and keep stirring until After the solid was dissolved, methyl iodide (4.0 mmol) was slowly added dropwise, and then stirred at room temperature for 2-6 h. After the reaction was completed, the solvent was removed under reduced pressure, ice water was added, and the solid was collected by filtration and recrystallized with absolute ethanol to obtain intermediate 2- Amino-5-methylmercapto-1,3,4-oxadiazole;
[0064] (3) 2-Amino-5-methylmercapto-1,3,4-oxadiazole (2.6mmol), potassium carbonate (3.9mmol) and toluene (20mL) were used as solvents, and put into a 100mL three-necked flask, and the solid After complete dissolution, add ...
Embodiment 3
[0065] Example 3: Synthesis of N-(5-(ethylthio)-1,3,4-thiadiazol-2-yl)-2-(trifluoromethyl)benzamide (compound number is A3), Include the following steps:
[0066] (1) with the (1) step of embodiment 1;
[0067] (2) Put 2-amino-5-mercapto-1,3,4-oxadiazole (4.0mmol), potassium carbonate (6.0mmol) and DMF (2mL) as solvent into a 100mL three-necked flask, and keep stirring until After the solid was dissolved, ethyl bromide (4.0 mmol) was slowly added dropwise, and then stirred at room temperature for 2-6 h. After the reaction was completed, the solvent was removed under reduced pressure, ice water was added, and the solid was collected by filtration and recrystallized from absolute ethanol to obtain intermediate 2 -Amino-5-ethylmercapto-1,3,4-oxadiazole;
[0068] (3) Put 2-amino-5-ethylmercapto-1,3,4-oxadiazole (2.6mmol), potassium carbonate (3.9mmol) and toluene (20mL) into a 100mL three-necked flask as solvent, and wait until the solid After complete dissolution, add 2-(trifl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com