New synthetic method of PET imaging agent L-5-<18>FETP
A reaction, hydroxytryptophan technology, applied in the field of medical imaging agents, can solve problems such as inability to use clinically, inability to effectively distinguish tumor lesions from normal tissues, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Non-radioactive labeling
[0109] Cold test standard O-(2-[ 19 F]-fluoroethyl)-L-5-hydroxytryptophan (L-5- 19 FETP) preparation
[0110] Step 1: Preparation of 1,2-ethylene glycol p-toluenesulfonate
[0111]
[0112] Add 6.2g (0.1mol) of ethylene glycol and 32mL of anhydrous pyridine into a 100mL three-necked round-bottomed flask, and cool to below 0°C in an ice-salt bath. Add 38.2g (0.2mol) of p-toluenesulfonyl chloride in batches under stirring. During the whole reaction process, continuously suck out the ice water in the ice-salt bath and put in new ice cubes to ensure that the reaction temperature remains below 0°C. When the portionwise addition of p-toluenesulfonyl chloride was complete, the reaction was continued (temperature less than 5°C) for 6 hours with constant stirring. After the reaction, the reaction mixture was poured into ice water, and a white solid was precipitated, filtered, washed with water, and dried to obtain 20.2 g o...
Embodiment 2
[0140] Embodiment 2: Optimization of thermal reaction test conditions
[0141]In order to make the reaction complete under the existing conditions, obtain higher radiochemical yields, and have better reproducibility, the present invention respectively adjusts the reaction conditions such as: the preparation of the stock solution, the temperature of the reaction, the speed of ventilation, the reaction The acidity and alkalinity of the solution, the reaction time and the concentration of the reaction solution were investigated.
[0142] 1: Preparation of stock solution
[0143] stock solution (acetonitrile / H 2 O / K 2 CO 3 / K 2.2.2 ), the stock solution is used to [ 18 F]F - It is eluted from the QMA resin column, then evaporated to remove water, and undergoes a nucleophilic reaction with the reaction precursor. According to literature reports, the proportion of water in the stock solution is within a relatively large range. In the fluorine nucleophilic substitution re...
Embodiment 3
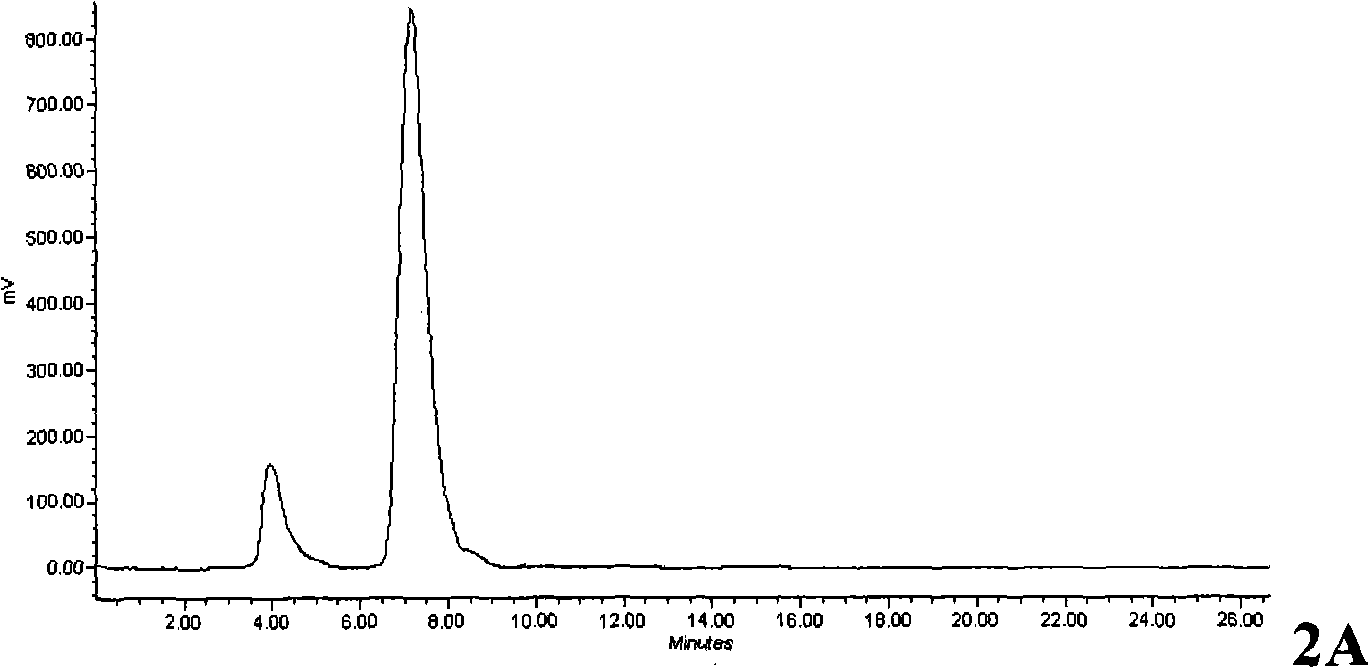
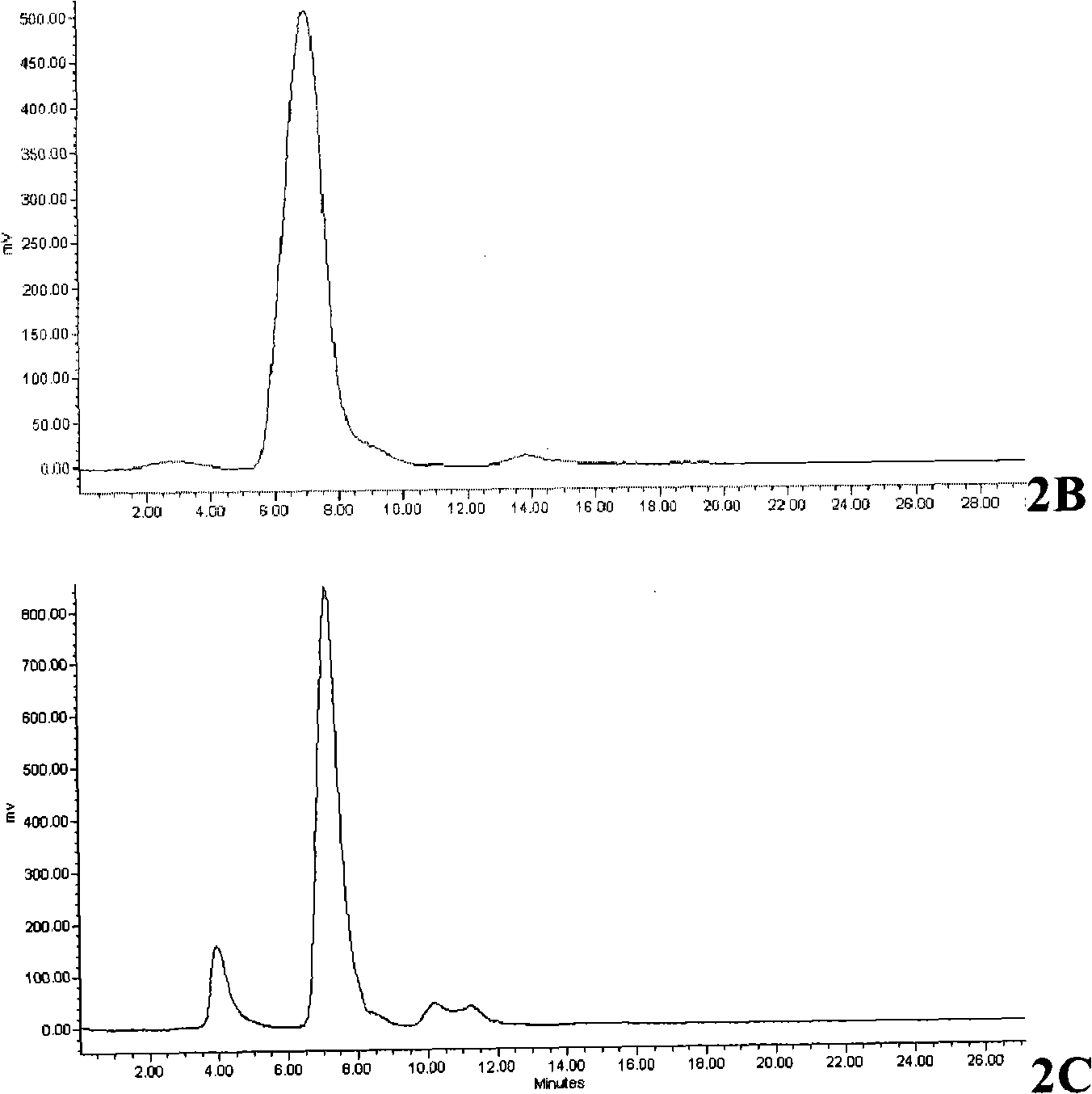
[0164] Example 3: Radiolabeling
[0165] Thermal experiment target product O-(2-[ 18 F]-fluoroethyl)-L-5-hydroxytryptophan (L-5- 18 FETP) preparation of
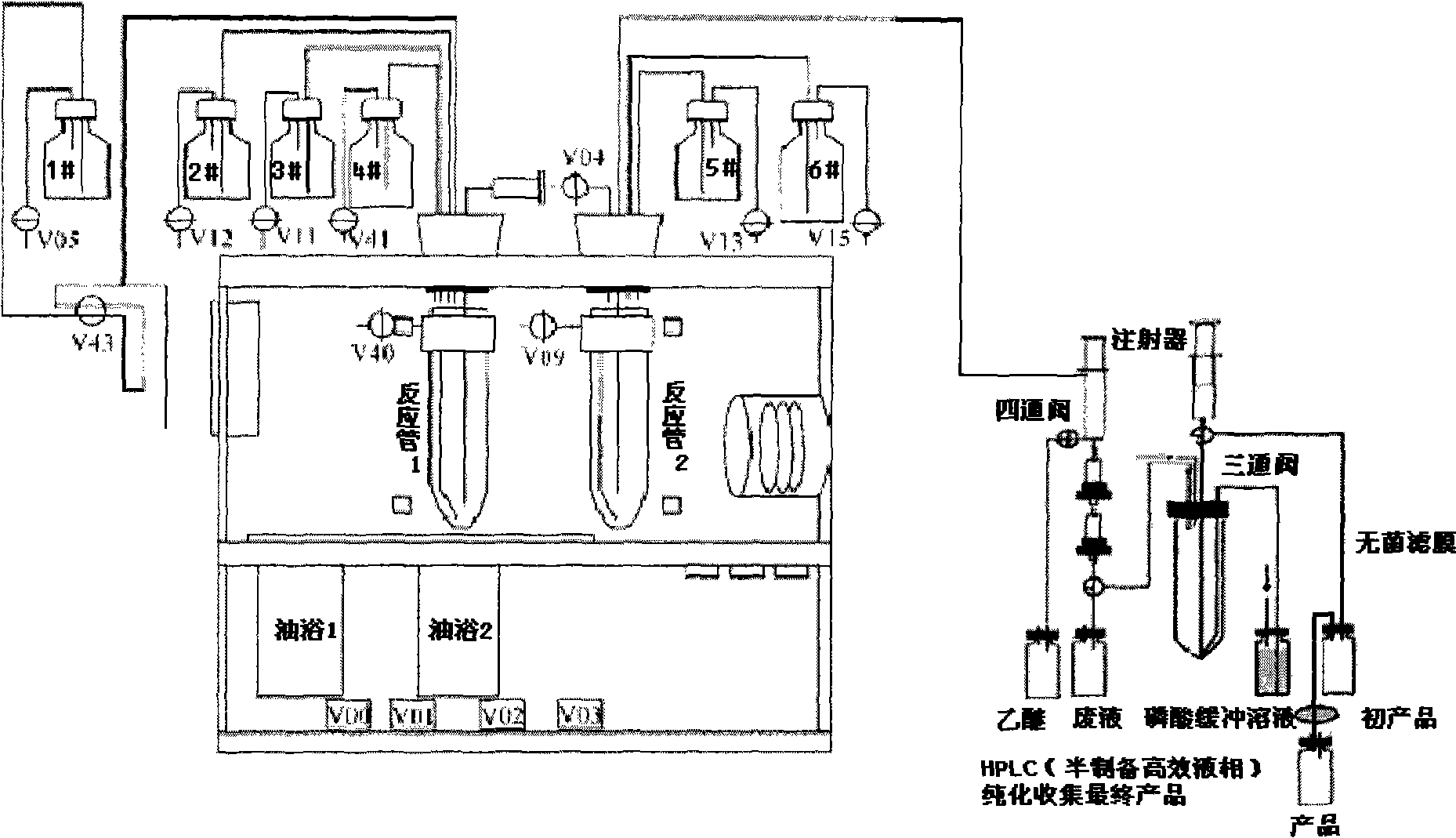
[0166] 1:[ 18 F] Modification of FDG Computer Controlled Chemical Synthesis Unit (CPCU)
[0167] The original laboratory [ 18 F] FDG computer-controlled chemical synthesis module (CPCU) is produced by Simens / CTI for [ 18 F] FDG double-tube method is used for synthesis, the automatic synthesis program is limited and cannot be changed, and there are only 6 sample bottles for use in the device, so it is limited to be used for the synthesis of other and [ 18 F] Imaging agents with different FDG synthesis methods or steps. Used in the present invention to synthesize L-5- 18 The two methods of FETP (single-tube method and double-tube method) are relatively [ 18 F]FDG is more complicated, so it must be modified according to the needs, that is, add a syringe and a pipeline as a sample bottle, and add a small sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com