Treatment of cardiac dysfunction and heart failure with reduced ejection fraction with compound (r)-4-(1-((3-(difluoromethyl)-1-methyl-1h-pyrazol-4-yl) sulfonyl)-1-fluoroethyl)-n-(isoxazol-3-yl) piperidine-1-carboxamide
A heart failure, dysfunction technique applied to the use of the compound (R)-4-(1-((3-(difluoromethyl)-1-methyl-1H-pyrazol-4-yl)sulfonyl )-1-fluoroethyl)-N-(isoxazol-3-yl)piperidine-1-carboxamide in the field of treatment of systolic dysfunction and heart failure with reduced ejection fraction, capable of addressing non-approved cardiac Exhaustion and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
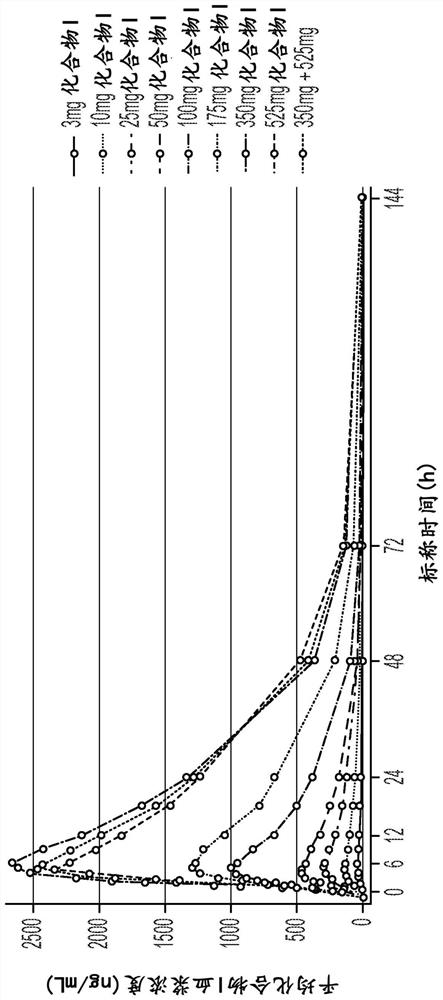
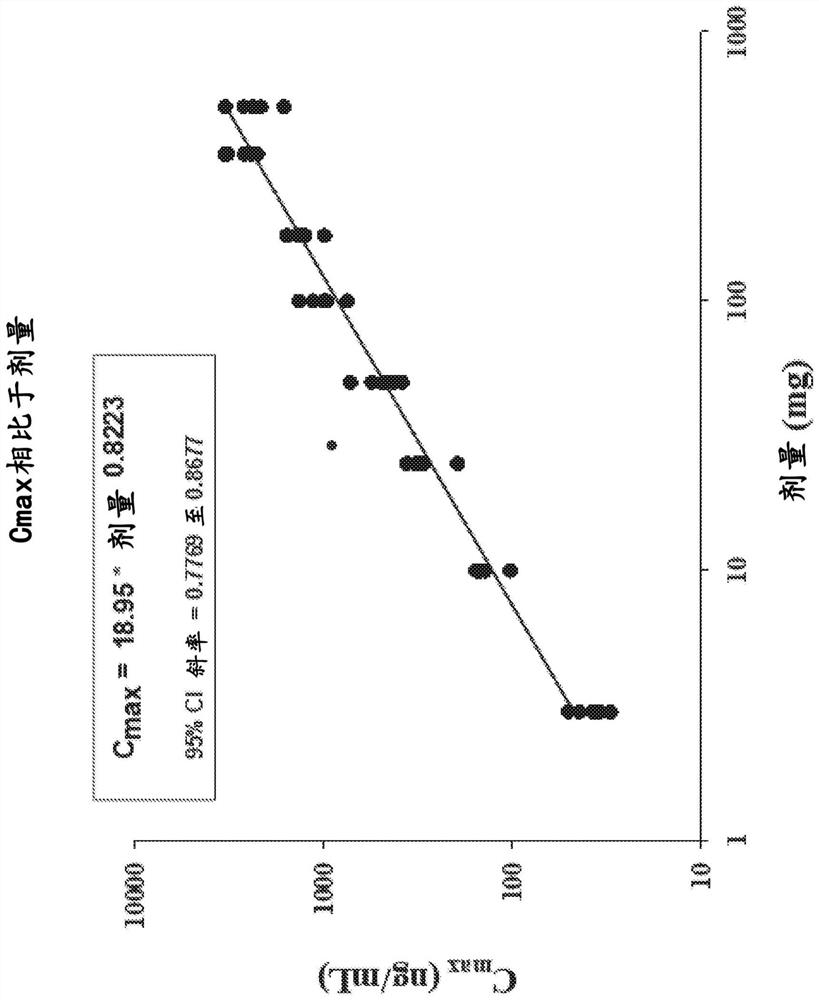
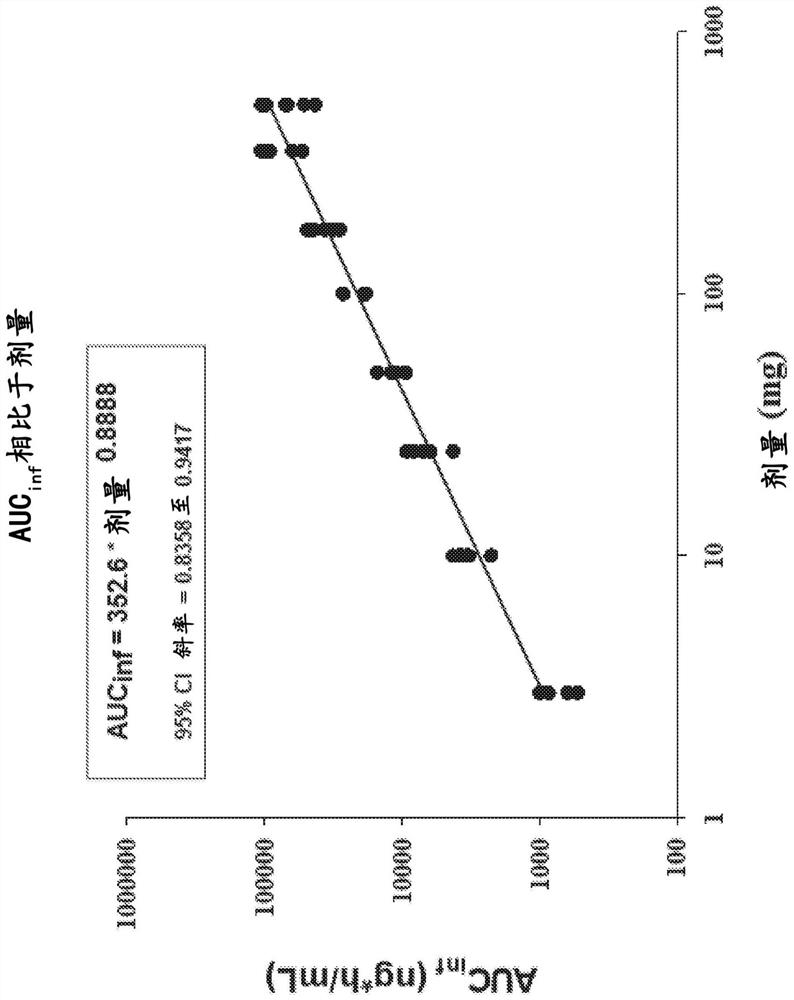
[0189] Example 1: Randomized, Placebo-Controlled Study of Safety, Tolerability, Preliminary Pharmacokinetics and Pharmacodynamics of Single Ascending Oral Doses of Compound I in Healthy Adult Volunteers
[0190] This example illustrates the first study of Compound I in humans. Based on the mechanism of action of Compound I, Compound I may provide targeted therapy for patients suffering from DCM caused by genetic or non-genetic mechanisms. The study was a randomized, double-blind, placebo-controlled, sequential group, single ascending (oral) dose study in healthy subjects aged 18-55 years. Eight dosing cohorts were enrolled, each containing 8 healthy subjects. Within each cohort, subjects were randomized 6:2 to Compound 1:placebo.
[0191] Materials and methods
[0192] Research design
[0193] Subjects stayed at the clinical site for up to 5 days and 4 nights, from Day -1 (the day before dosing) to Day 4, and received a single dose of Compound I or placebo on Day 1. ECG t...
Embodiment 2
[0318] Example 2: An open-label, pilot, randomized two-phase crossover study evaluating the effect of food on a 25 mg tablet formulation of Compound I at a dose of 200 mg in healthy adult volunteers
[0319] This example illustrates the clinical study used to establish the effect of a high fat, high calorie meal on the PK profile of Compound I in healthy volunteers compared to drug administered in the fasted state. The study also sought to determine the safety and tolerability following a single oral dose of Compound I in healthy volunteers in the fed and fasted state. Measurements of PK, PD and other clinical parameters were performed as described in Example 1 above.
[0320] Materials and methods
[0321] Research design
[0322] This study was an open-label, randomized, two-period crossover study in healthy volunteers aged 18-55 years. Subjects were screened up to 28 days prior to the first treatment period. Subjects were admitted to the clinical site on Day -1 of Perio...
Embodiment 3
[0369] Example 3: Safety, Tolerability, Preliminary Pharmacokinetics and Pharmacodynamics of Single and Multiple Ascending Oral Doses of Compound I in Patients with Stable HFrEF Randomized, Double-Blind, Placebo-Controlled, Two-Part, Adaptive Design Study
[0370] This example illustrates a preliminary safety and tolerability study establishing single and multiple ascending oral doses of Compound I in ambulatory patients with stable heart failure with reduced ejection fraction (HFrEF). Key eligibility criteria included stable HFrEF of ischemic or non-ischemic origin treated with guideline-directed medical therapy (EF at screening initially required 20% to 45%, and subsequently changed to 15% to 35% upon revision). Subjects with active ischemia or severe or valvular heart disease were excluded. The study also aimed to (1) establish the preliminary human PK of Compound I after single and multiple ascending oral doses of Compound I in patients with HFrEF; Changes in left ventri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com