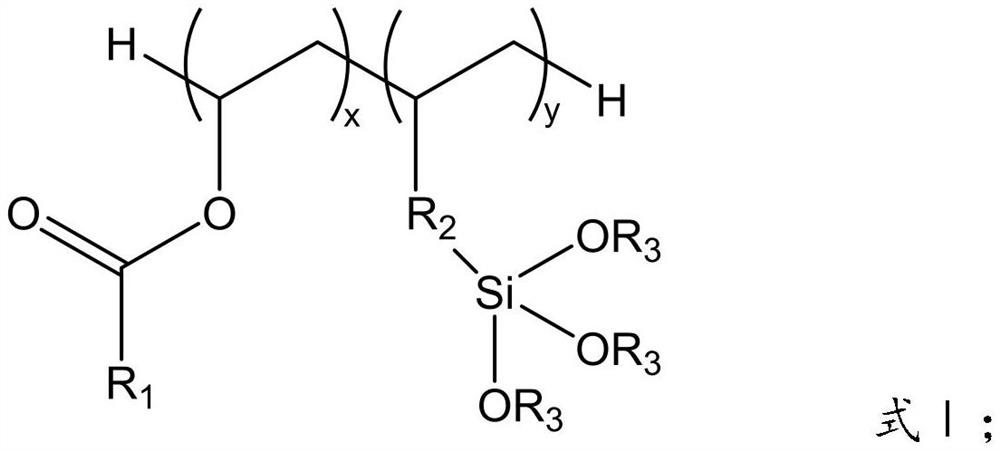
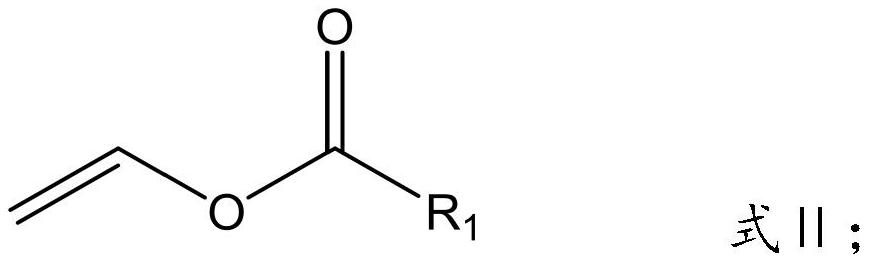
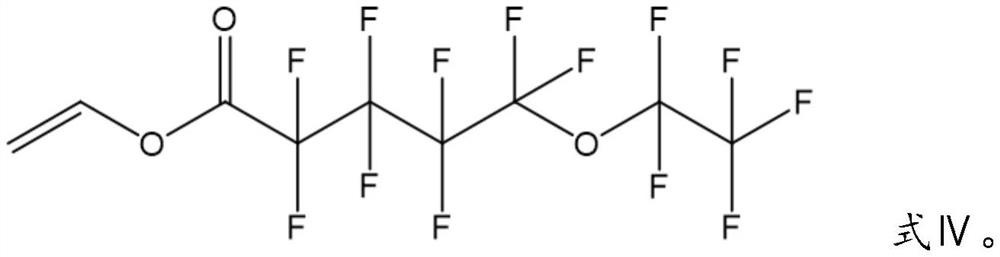
Silane-modified fluoroethyl ester polymer used as lithium battery binder and preparation method thereof
A silane modification and silane compound technology, which is applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of poor rate performance of silicon negative electrodes, failure to meet commercial needs, poor high temperature resistance of batteries, etc., to improve rate performance and effectively Good for electrochemical performance and strong chemical corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]First, the weighed 21.42 g (0.05 mol) perfluorooctanoate, 20.41 g (0.1 mol) propylenetriethoxysilane and 50 ml of butyl formate solvent were added to the four flasks and stirred; After heating to 60 °C and stabilized, the initiator azo diisobutyronitrile and 10 ml of butyrate solvent of 0.1255 g (0.0008 mol) were mixed evenly and added to the four flasks dropwise; After 12h of the reaction, the solvent was transferred to a rotary evaporator to remove the solvent, and finally the precipitate was washed with n-hexane, and the precipitate was dried to give the silane-modified fluoroethyl ester polymer.
[0039] Then, the silicon anode lithium ion buckle type half battery is assembled using the above polymer binder: according to the commercial silicon particles, carbon black, binder mass ratio of 8:1:1, and add N-methylpyrrolidone solvent to mix evenly, coated copper foil collector, vacuum 100 °C drying for 8 hours, sliced as a 12mm disc, assembled in the glove box battery, plac...
Embodiment 2
[0042] First, the weighed 21.42 g (0.05 mol) perfluorooctanoate, 16.41 g (0.1 mol) ethoxytrimethoxysilane and 50 ml of butyl formate solvent were added to a four-mouth flask and stirred; After heating up to 65 °C and stabilized, the initiator 0.1513 g (0.0009 mol) of azo diisobutyronitrile and 10 ml of butyl formate solvent were mixed evenly, and added to the four flasks dropwise; After 12h of the reaction, the solvent was transferred to a rotary evaporator to remove the solvent, and finally the precipitate was washed with n-hexane, and the precipitate was dried to give the silane-modified fluoroethyl ester polymer.
[0043] Then, the silicon anode lithium ion buckle type half battery is assembled using the above polymer binder: according to the commercial silicon particles, carbon black, binder mass ratio of 8:1:1, and add N-methylpyrrolidone solvent to mix evenly, coated copper foil collector, vacuum 100 °C drying for 8 hours, sliced as a 12mm disc, assembled in the glove box ba...
Embodiment 3
[0046] First, the weighed 31.40 g (0.05 mol) perfluorodecanoate vinyl, 8.21 g (0.05 mol) ethylene ethoxytrimethoxysilane and 50 ml of butyl formate solvent were added to a four-mouth flask and stirred; After heating up to 70 °C and stabilizing, the initiator azo diisobutyronitrile and 10 ml of butyrate solvent of 0.1983 g (0.0012 mol) were mixed evenly and added to the four flasks dropwise; After 11h of the reaction, the solvent was removed from the rotary evaporator, and finally the precipitate was washed with n-hexane, and the precipitate was dried to give the silane-modified fluoroethyl ester polymer.
[0047] Then, the silicon anode lithium ion buckle type half battery is assembled using the above polymer binder: according to the commercial silicon particles, carbon black, binder mass ratio of 8:1:1, and add N-methylpyrrolidone solvent to mix evenly, coated copper foil collector, vacuum 100 °C drying for 8 hours, sliced as a 12mm disc, assembled in the glove box battery, place...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com