Method for preparing 18F-FET
A 18F-FET, volume concentration technology, applied in chemical instruments and methods, cyanide reaction preparation, organic compound preparation, etc., can solve the problems of low efficiency, low chemical purity, long synthesis time, etc., to reduce waste The production amount, the improvement of chemical purity, and the effect of improving synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
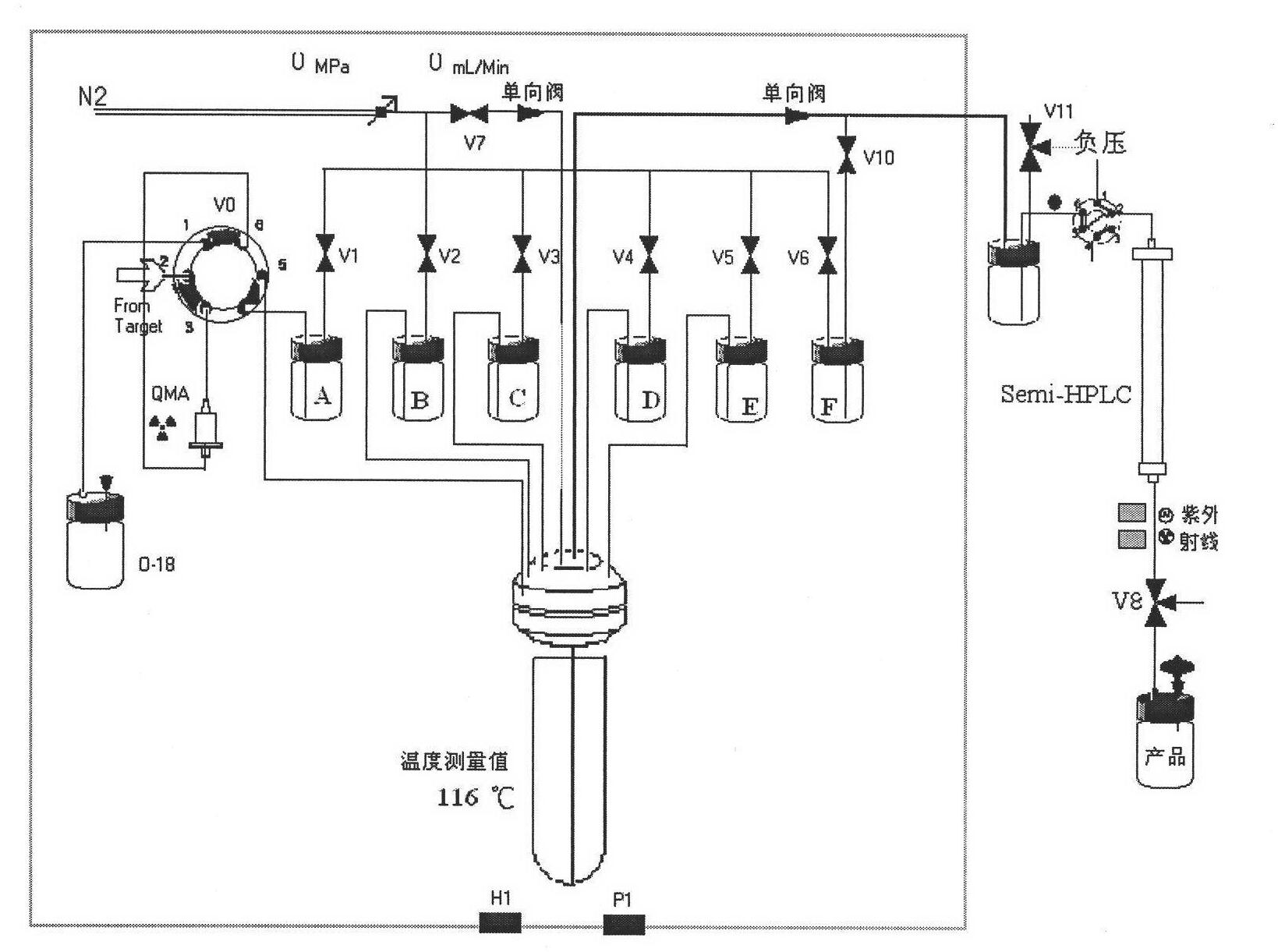
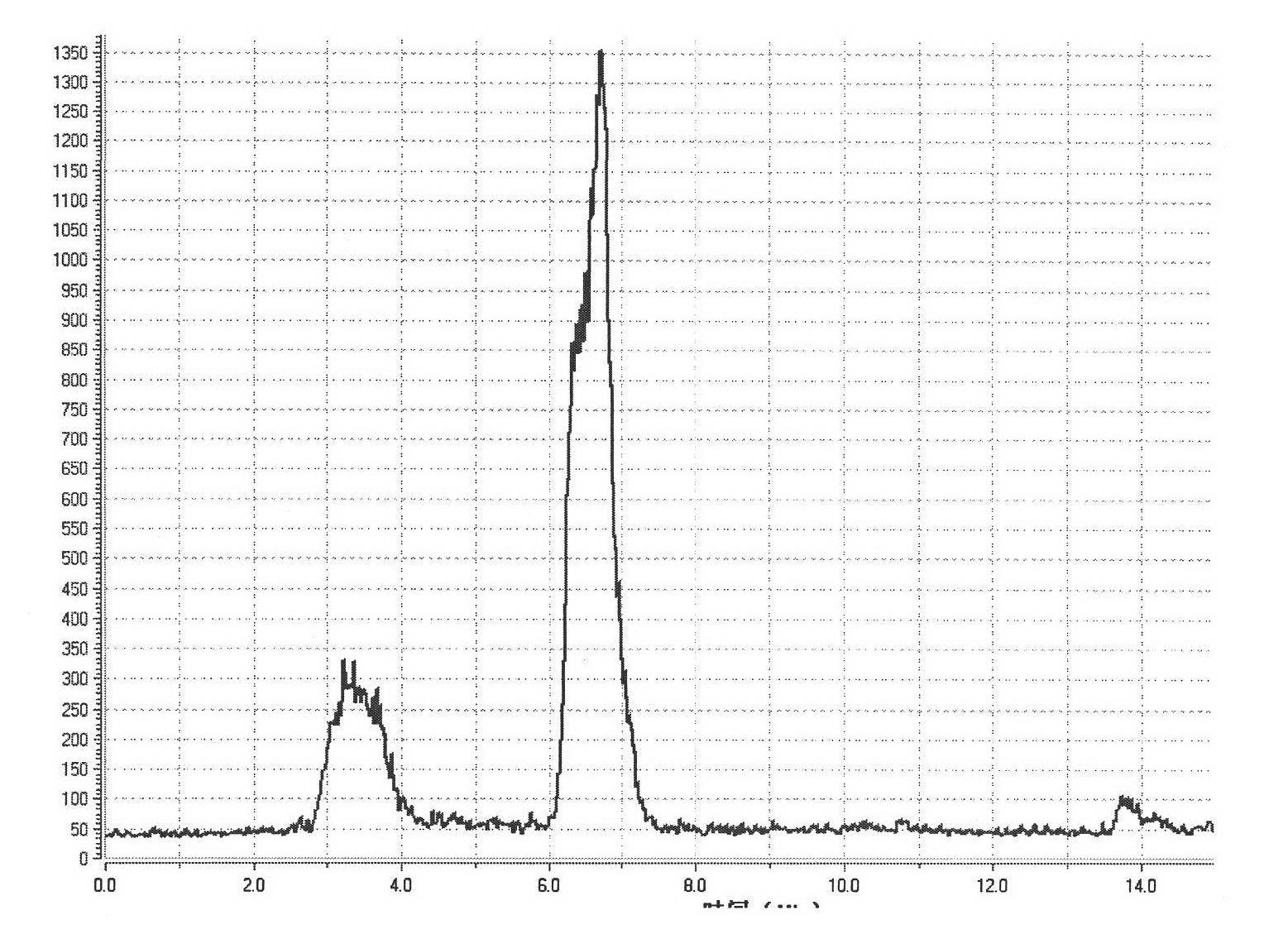
[0023] in the attached figure 1 The 18F-FET is synthesized on the common FDG module shown, and the radioactive F-18 ions produced by the accelerator are passed to the QMA column of the FDG module through nitrogen gas, K2.2.2 / K2CO 3 Acetonitrile aqueous solution (bottle A) pour F-18 from the QMA column into the reaction tube, remove acetonitrile under heating and open conditions, add 2 mL of acetonitrile (bottle B) to the reaction tube again, and remove acetonitrile. Add 0.6 mL of DMSO solution containing 10 mg of ethylene glycol p-toluenesulfonate (bottle C) into the reaction tube, heat to 85 ° C for 5 min, and add 0.6 mL of DMSO containing 9 mg of sodium tyrosine / 40 μL of 10% KOH to the reaction tube solution (bottle D), heat the reaction tube to 120°C for 5 minutes. Add 3 mL of 8% ethanol in water to the reaction tube, pass the mixed solution to a semi-prepared HPLC column for separation, the separation column is a C-18 column, and the mobile phase is an aqueous solution of...
Embodiment 2
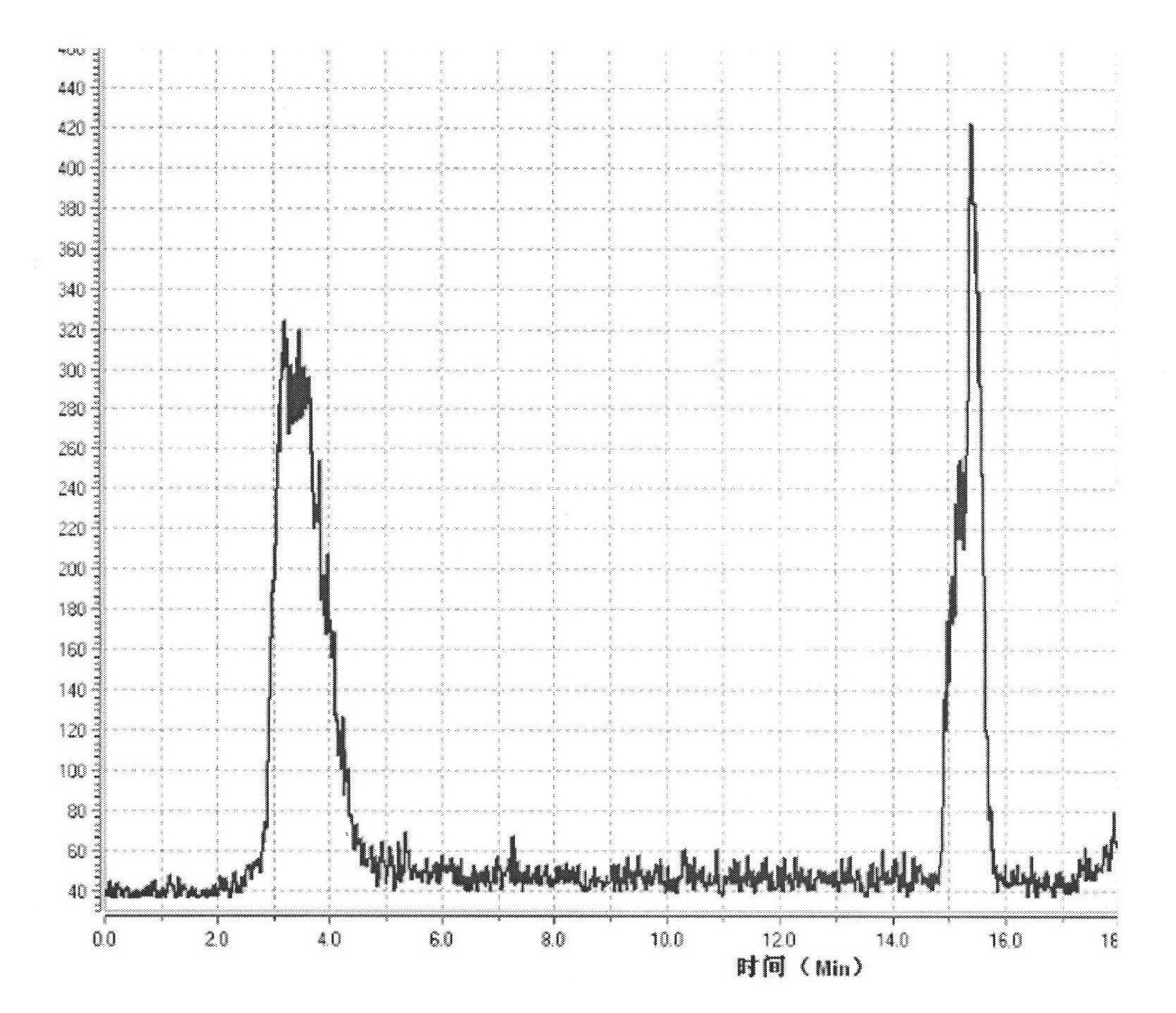
[0025] in the attached figure 1 18F-FET is synthesized on the common FDG module shown, but the semi-preparative HPLC mobile phase is changed to improve the chemical purity of the product: the radioactive F-18 ion produced by the accelerator is passed to the QMA column of the FDG module through nitrogen, K2.2.2 / K2CO 3 Acetonitrile aqueous solution (bottle A) pour F-18 from the QMA column into the reaction tube, remove acetonitrile under heating and open conditions, add 2 mL of acetonitrile (bottle B) to the reaction tube again, and remove acetonitrile. Add 0.6 mL of acetonitrile solution containing 10 mg of ethylene glycol p-toluenesulfonate (bottle C) into the reaction tube, heat to 85 ° C for 5 minutes, blow nitrogen to remove acetonitrile to 0.1 mL, add 0.6 mL of 9 mg of tyrosine to the reaction tube NaCl / 40 μL 10% KOH in DMSO solution (bottle D), heat the reaction tube to 120°C for 4 minutes, mix with nitrogen gas, and then heat for 1 minute. Add 3mL of 8% ethanol in wat...
Embodiment 3
[0027] in the attached figure 1 The 18F-FET is synthesized on the common FDG module shown, and the radioactive F-18 ions produced by the accelerator are passed to the QMA column of the FDG module through nitrogen gas, K2.2.2 / K2CO 3Acetonitrile aqueous solution (bottle A) pour F-18 from the QMA column into the reaction tube, remove acetonitrile under heating and open conditions, add 2 mL of acetonitrile (bottle B) to the reaction tube again, and remove acetonitrile. Add 0.6 mL of acetonitrile solution containing 10 mg of ethylene glycol p-toluenesulfonate (bottle C) into the reaction tube, heat to 85 ° C for 5 min, and directly add 0.6 mL of 9 mg sodium tyrosine / 40 μL of 10% KOH to the reaction tube DMSO (bottle D), heat the reaction tube to 120°C for 5 min. Add 3 mL of 8% ethanol in water to the reaction tube, pass the mixed solution to a semi-prepared HPLC column for separation, the separation column is a C-18 column, and the mobile phase is an aqueous solution of 6% ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com