Positron emission tomography (PET) diagnostic radioactive drug and preparation method thereof
A radiopharmaceutical and positron tomography technology, applied in the directions of in vivo radioactive preparations, pharmaceutical formulations, preparations for in vivo experiments, etc., can solve the problems of insignificant advantages, inability to meet the requirements of PBRPET imaging research, etc., to reduce in vivo degradation, The effect of increasing steric hindrance and providing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
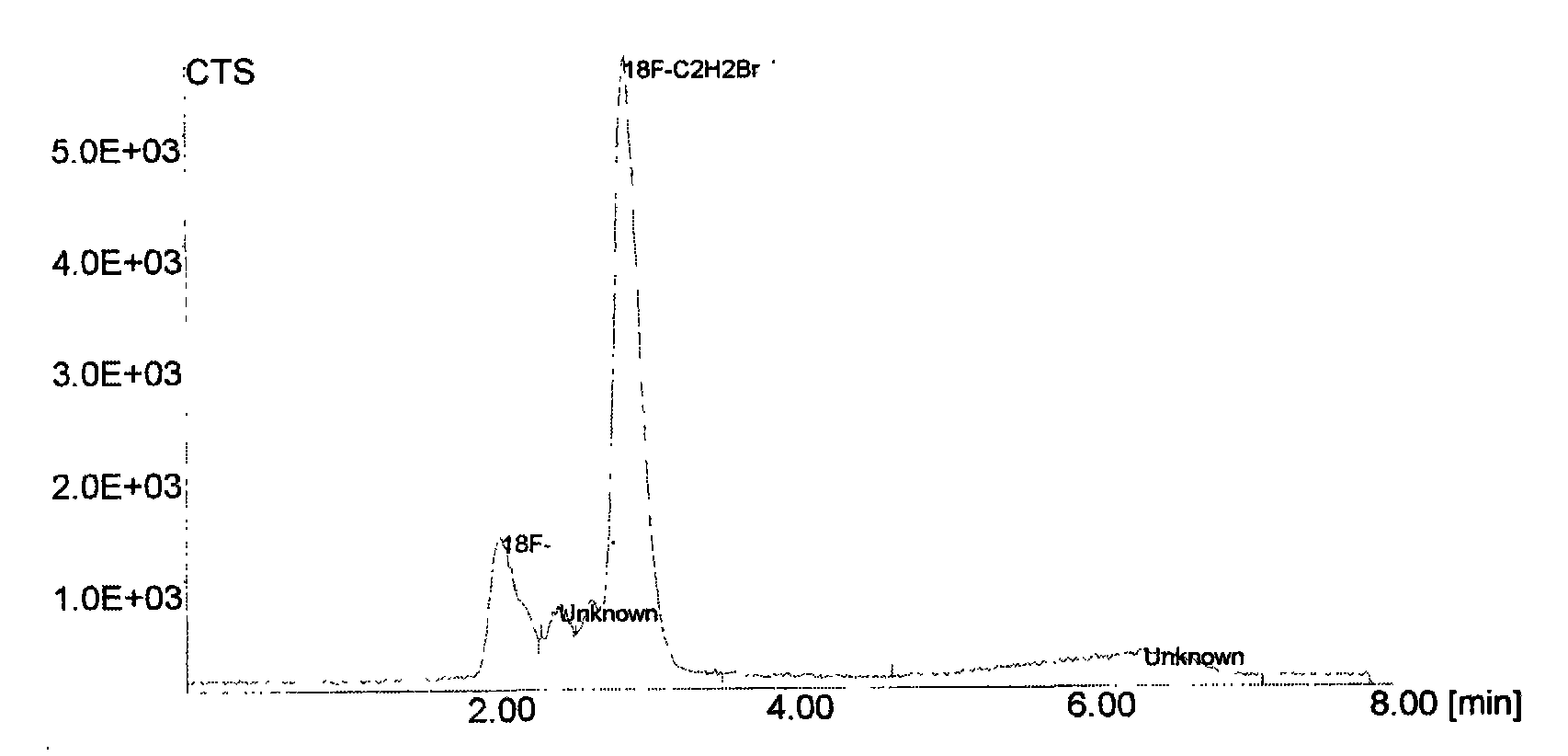
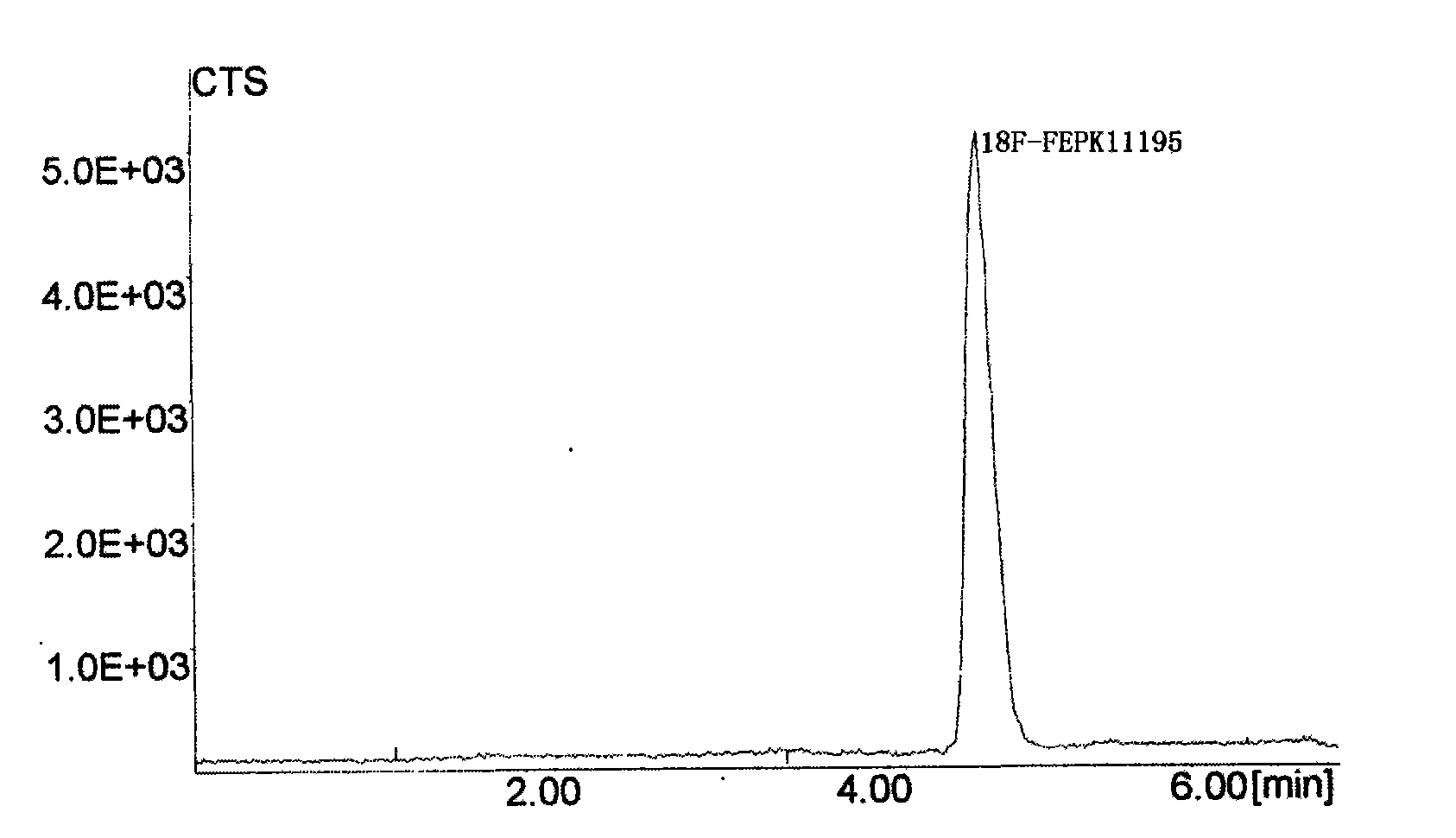
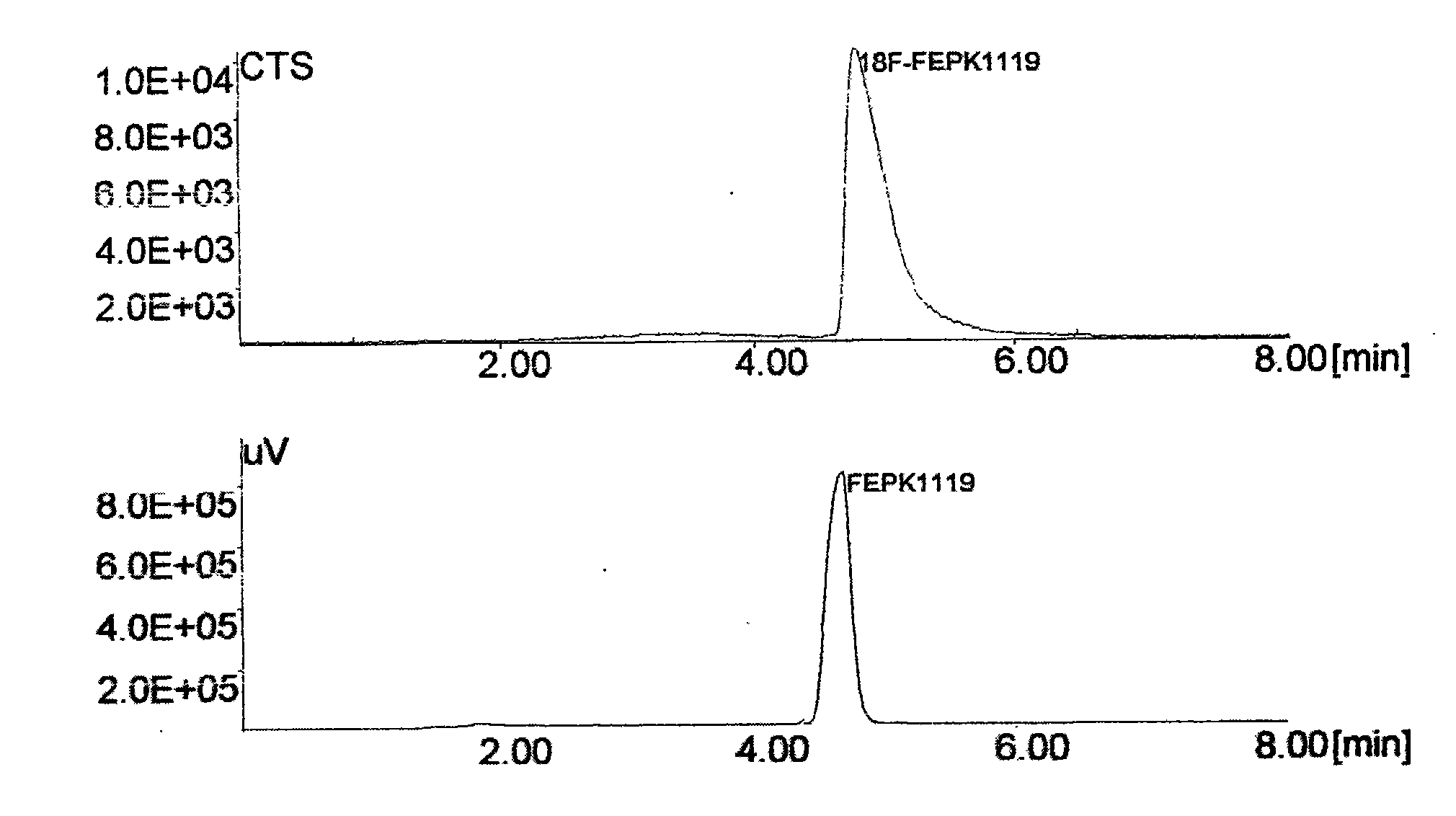
Image
Examples
Embodiment 1
[0026] N-[ 18 F] the preparation method of fluoroethyl-N-(1-methylpropyl)-1-(2-chlorophenyl) isoquinoline-3-carbamoyl, its preparation steps are as follows:
[0027] (1) Produced by accelerator 18 f - After being captured by the anion column QMA (Waters), it is eluted by the acetonitrile solution of sodium bicarbonate and cryptane K2.2.2, and the eluate is heated to azeotrope with acetonitrile to remove water, the azeotropic temperature is 82°C, and the time is 230 seconds. The cyclotron used may be produced by GE Company of the United States, and the model is PETtrace. Wherein, the component content of the acetonitrile solution of potassium carbonate and cryptane K2.2.2 is as follows:
[0028] Reagent name
Dosage
K 2 CO 3
30mg
pure water
1ml
K2.2.2
130mg
HPLC grade acetonitrile
10ml
[0029] (2) After cooling, add 0.25ml of 1,2-dibromoethane and acetonitrile to the residue, react at 85°C for 5min, cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com