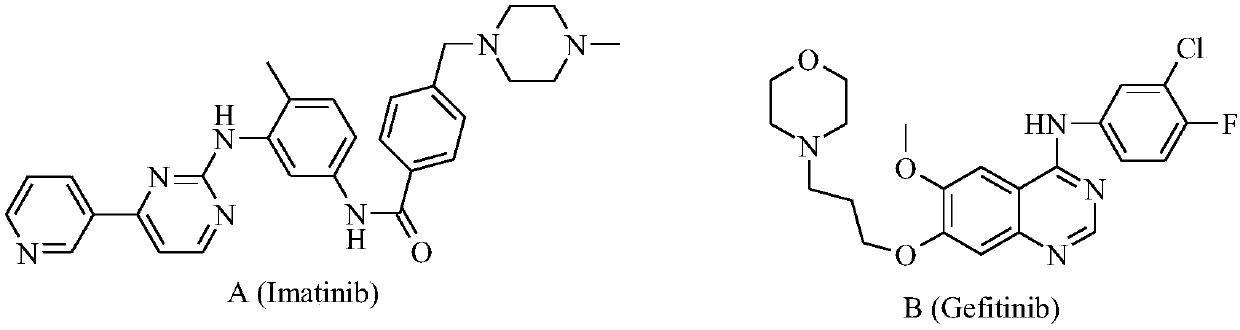
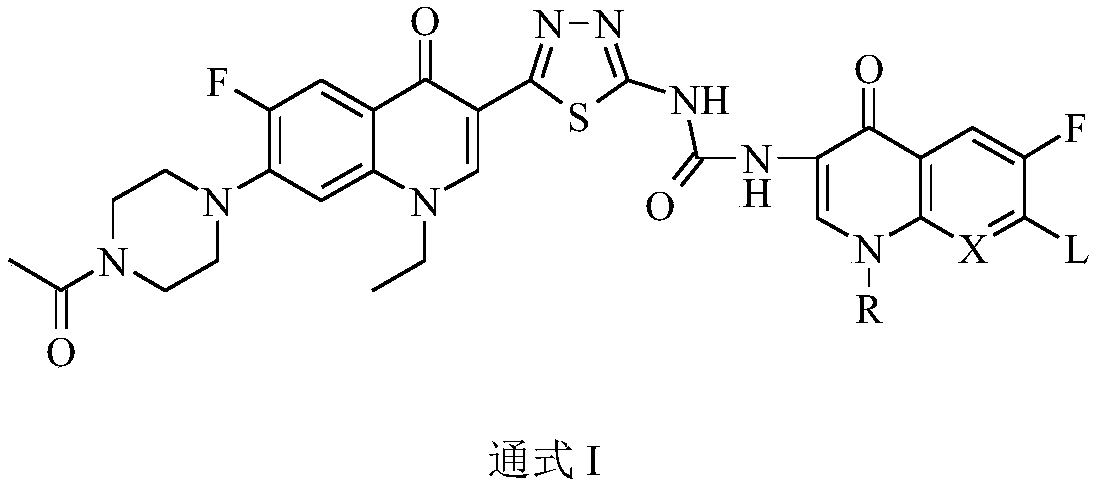
Preparation and application of bis-fluoroquinolone thiadiazole ureas N-acetyl norfloxacin derivative
A technology of fluoroquinolone thiadizuron and acetyl norfloxacin, which is applied to the preparation of such derivatives and the application of antitumor drugs. In the field of object design, to achieve the effect of innovative structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
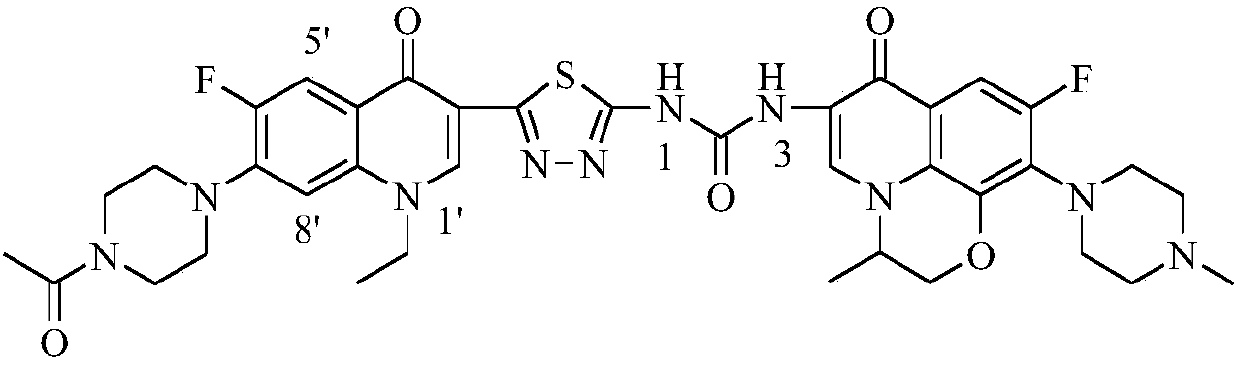
[0034] 1-{2-[1-Ethyl-6-fluoro-7-(4-acetylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-1,3,4 -Thiadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)-quinoline-4 (1H)-ketone-3-yl]-urea (I-1), its chemical structural formula is:
[0035]
[0036] The preparation method of the bis-fluoroquinolone thiadizuron of the present embodiment is: get ofloxacin hydroxamic acid (1 ") 1.0g (2.7mmol) and suspend in 25mL acetonitrile, add carbonyldiimidazole (CDI) 0.67g (4.1mmol), stirring at room temperature until the material is dissolved. Then add N-acetyl norfloxacin C-3 thiadiazolamide V intermediate 1.12g (2.7mmol), stir in a water bath at 55-60°C for 15 hours. Leave overnight, filter The resulting solid was collected and washed with acetonitrile. The crude product was recrystallized from a DMF-ethanol mixed solvent to obtain a light yellow crystal (I-1), with a yield of 68%, m.p.232-234°C. 1 H NMR (400MHz, DMSO-d 6 )δ: 11.55 (brs, 1H, NH), 9.46 (s, 1H, NH), 9.17, 8...
Embodiment 2
[0038] (S)-1-{2-[1-Ethyl-6-fluoro-7-(4-acetylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-1 ,3,4-Thiadiazol-5-yl}-3-[6-fluoro-7-(4-methylpiperazin-1-yl)-8,1-(1,3-oxopropyl)- Quinoline-4(1H)-ketone-3-yl]-urea (I-1), its chemical structural formula is:
[0039]
[0040] The preparation method of the bis-fluoroquinolone thiadizuron of this embodiment is: take levofloxacin hydroxamic acid (2″) 1.0g (2.7mmol) and suspend in 25mL acetonitrile, add carbonyldiimidazole (CDI) 0.60g (3.7mmol ), stirred at room temperature until the material was dissolved. Then 1.12 g (2.7 mmol) of N-acetyl norfloxacin C-3 thiadiazole amide V intermediate was added, and stirred in a water bath at 55-60° C. for 10 hours. Placed overnight, and the resulting The solid was washed with acetonitrile. The crude product was recrystallized from ethanol to obtain a light yellow crystal (I-2), with a yield of 54%, m.p.218-220°C. 1 H NMR (400MHz, DMSO-d 6 )δ:11.57(brs,1H,NH),9.44(s,1H,NH),9.17,8.96(2s,2H,2×2′-H),8...
Embodiment 3
[0042] 1-{2-[1-Ethyl-6-fluoro-7-(4-acetylpiperazin-1-yl)-quinolin-4(1H)-one-3-yl]-1,3,4 -Thiadiazol-5-yl}-3-[6,7-difluoro-1,8-(1,3-oxopropyl)-quinolin-4(1H)-one-3-yl]-urea (I-3), its chemical structural formula is:
[0043]
[0044] The preparation method of the bis-fluoroquinolone thiadiazole of the present embodiment is: take 1.0 g (3.4 mmol) of oxyfluorocarboxylic acid hydroxamic acid (3″) and suspend it in 25 mL of acetonitrile, add 0.82 g of carbonyldiimidazole (CDI) (5.1mmol), stirring at room temperature until the material is dissolved. Then add 1.41g (3.4mmol) of N-acetyl norfloxacin C-3 thiadiazole amide V intermediate, and stir in a water bath at 55-60°C for 20 hours. Place overnight, filter The resulting solid was collected and washed with acetonitrile. The crude product was recrystallized from a DMF-ethanol mixed solvent to obtain a light yellow crystal (I-3), with a yield of 72%, m.p.226-228°C. 1 H NMR (400MHz, DMSO-d 6 )δ:11.54(brs,1H,NH),9.43(s,1H,NH),9.12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com