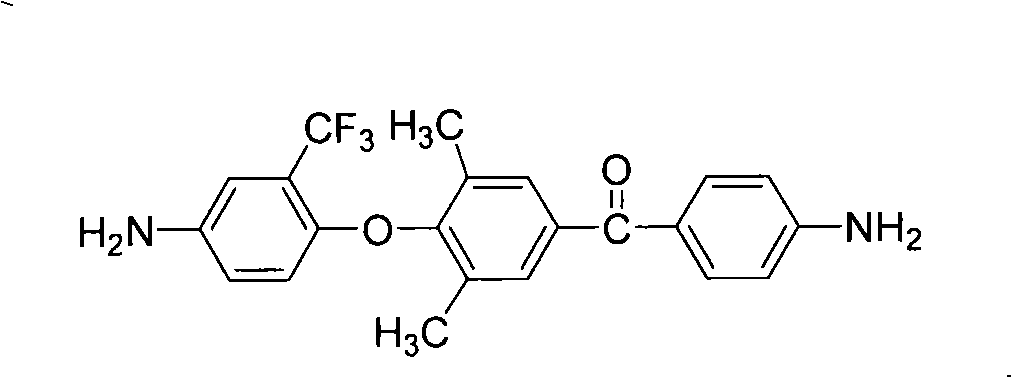
Unsymmetrical fragrant diamine containing fluorine, preparation and application in synthesizing polyimide thereof
A technology of aromatic diamine and polyimide, which is applied in the field of aromatic diamine, can solve the problems of high melting temperature and limited application of polyimide, and achieve the effect of excellent comprehensive performance and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of fluorine-containing asymmetric aromatic diamine
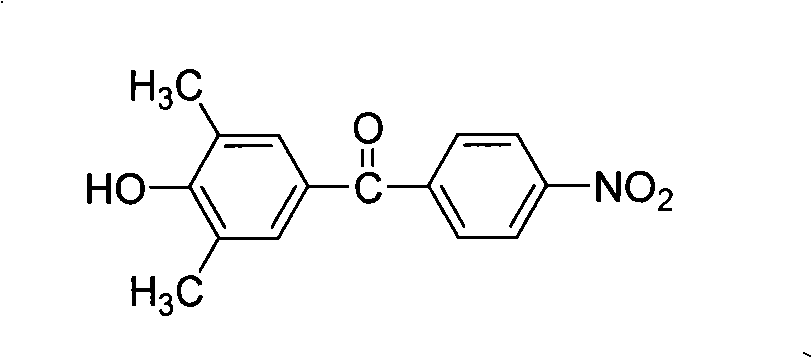
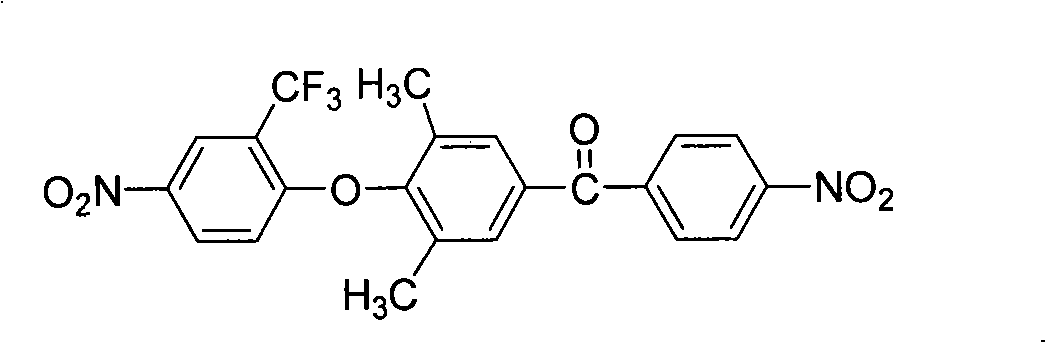
[0044] ①At 0~5℃, add 12.2g (0.1mol) 2,6-dimethylphenol and 28.0g (0.21mol) aluminum trichloride to 250ml containing 120ml 1,2-dichloroethane respectively In the three-neck flask, after fully stirring for half an hour, add 18.6g (0.1mol) p-nitrobenzoyl chloride in batches, stir fully for one hour, then raise the temperature to 10-20°C, react for 4-6 hours, and then pour 1000ml of dilute hydrochloric acid The reaction was completed by settling in the aqueous solution. After the precipitate was steam distilled, it was repeatedly washed with methanol / water (volume ratio 1:1) and recrystallized with ethanol to obtain a light yellow solid powder (4'-hydroxyl-3',5 '-Dimethylbenzene)-(4-nitrophenyl)methanone, the yield is about 80-90%, and the melting point is 214-215°C. FT-IR(KBr)v / cm -1 : 3434(O-H), 1655(C=O), 1523, 1350(-NO 2 ). 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.48 (s, 1H), 8.38 (d, 2H), 7.99 (d, ...
Embodiment 2
[0047] The preparation of embodiment 2 polyimide
[0048] Add 0.4004g (0.001mol) of asymmetric fluorine-containing aromatic diamine and 0.3102g (0.001mol) of diphenyl ether tetra-acid dianhydride monomer into a 50ml three-necked round-bottomed flask that is dry and ventilated with nitrogen, and then add 8ml of m-formazine Phenol and 0.1ml isoquinoline, heat the reaction system to 100-120°C for 2-5 hours, then raise the temperature to 190-200°C for about 15 hours, cool to 120°C, pour the reaction solution into methanol to settle to obtain white fibers The polymer sample was filtered and washed twice with boiling water, and the polymer sample was vacuum-dried at 150°C. FT-IR(film)v / cm -1 : 1781, 1728(C=O), 1371(C-N), 1237(C-O-C), 1127(C-F). 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.06-8.09 (d, 2H), 7.95-7.97 (d, 3H), 7.56-7.69 (d, 9H), 6.77-6.79 (d, 1H), 2.18 (s, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com