A kind of preparation method of methyl 2,2-difluoropiperate
A technology of methyl difluoropiperate and difluoropiperonyl fluoride is applied in 2 fields, can solve the problems of high cost, low total reaction yield, long steps and the like, and achieves the advantages of short synthesis route, reduced environmental pollution and complete reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
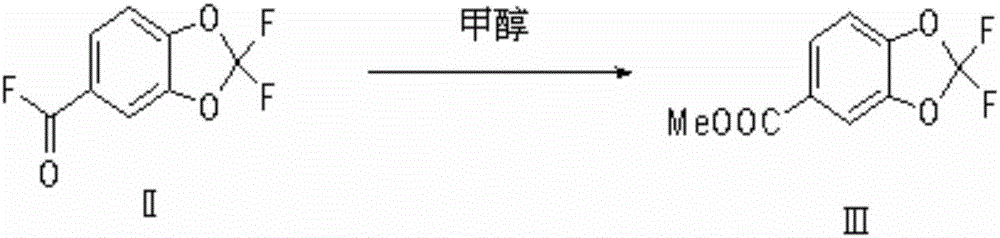
[0033] The invention provides a kind of preparation method of methyl 2,2-difluoropiperate, comprising the steps of:
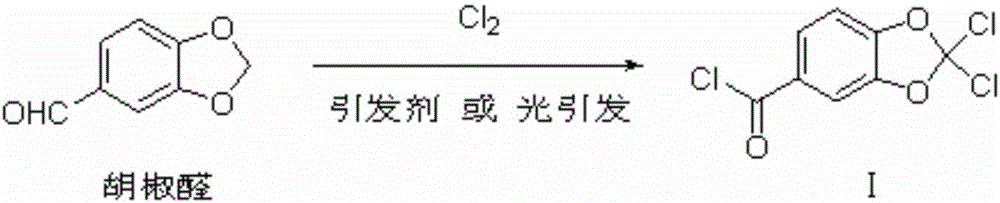
[0034] 1) Chlorination reaction: use piperonal as raw material, react with chlorine gas under the action of initiator or photoinitiation to prepare 2,2-dichloropiperoyl chloride, the reaction equation is as follows:
[0035]
[0036] Preferably, the specific method of the chlorination reaction is: when using an initiator to initiate the chlorination reaction, chlorine gas, piperonal dissolved in a reaction solvent, and the initiator are added to the reaction vessel simultaneously; when using light to initiate the chlorination reaction, Chlorine and piperonal dissolved in the reaction solvent are added to the reaction vessel simultaneously, and the reaction system is illuminated; the ratio of the speed of addition of chlorine to the speed of addition of piperonal in the reaction solvent is preferably 2-10 times the molar amount, more preferably 2-5:1, most pr...
Embodiment 1
[0062] 2, Preparation of 2-dichloropiperoyl chloride
[0063] After 300g piperonal, 0.3g azobisisobutyronitrile are mixed and dissolved with 600g p-chlorobenzotrifluoride, they are added dropwise in the reactor, while feeding chlorine (total amount 426g) at a speed of 71g / hour, and keep The internal temperature is 120-140°C. The piperonal solution was added dropwise in about 6 hours, and the reaction ended. Degassing under reduced pressure, then removing p-chlorotrifluorotoluene under reduced pressure, and then distilling under reduced pressure to collect fractions at 134-136°C / 1.4kPa to obtain 479g of 2,2-dichloropiperonyl chloride. The yield of the chlorination reaction was was 94.4%.
Embodiment 2
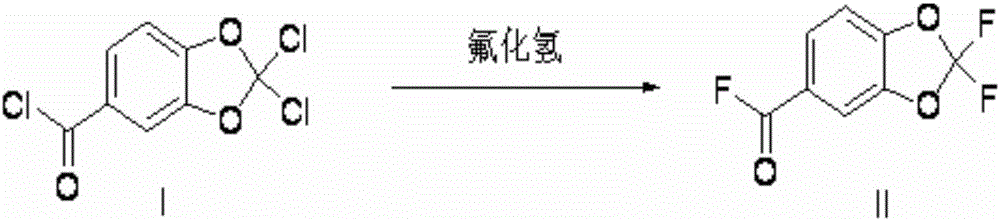
[0065] Preparation of 2,2-difluoropiperonyl fluoride
[0066] Put 479g of 2,2-dichloropiperonyl chloride into the polytetrafluoro reactor, raise the temperature inside the reactor to 40°C, and feed 120g of hydrogen fluoride under stirring, and then stir at 50-70°C for half an hour to react Finish. Measured by GC-MS method, the reaction product is 2,2-difluoropiperoyl fluoride. This reaction solution was directly used for the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com