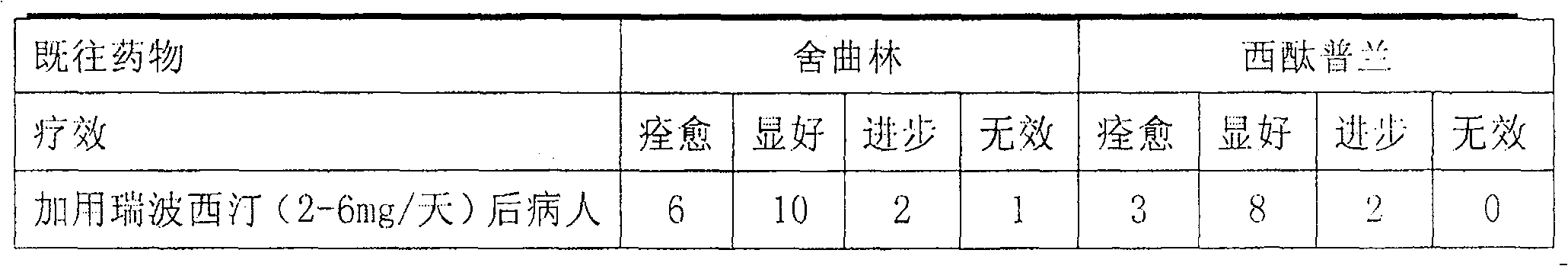
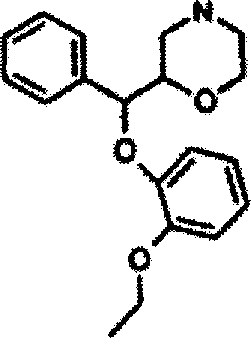
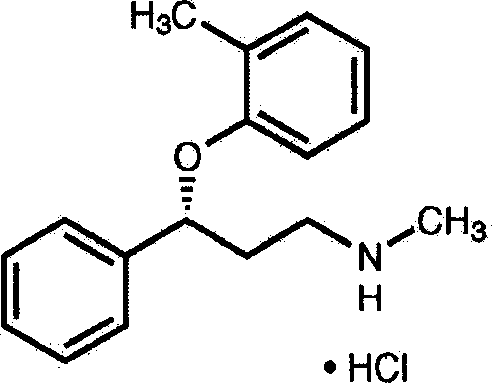
Medicinal composition for treating neurasthenia or somatic form disorders
A somatoform disorder and composition technology, applied in the field of pharmacy, can solve the problems of inaccurate curative effect, reduce the curative effect of various emotional symptoms, etc., and achieve the effects of stable and long-lasting effect, fast onset of effect, and reduction of incidence and intensity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1 Prepare reboxetine-sertraline compound tablets (1000 tablets) containing 1 mg reboxetine and 12.5 mg sertraline.
[0075] Formula: Reboxetine 1g
[0076] Sertraline 12.5g
[0077] Lactose 50g
[0078] Microcrystalline Cellulose 50g
[0079] Starch 10g
[0080] Sodium carboxymethyl starch 30g
[0082] Preparation method: pulverize 1g of reboxetine, 12.5g of sertraline, 50g of lactose, 50g of crystal cellulose and 10g of starch, mix them uniformly, make a soft material with 10% povidone ethanol solution, granulate, dry, For granulation, the granules with a water content of about 3% are uniformly mixed with magnesium stearate, and compressed into tablets with a tablet machine. Each of the prepared compound tablets contains 1 mg of reboxetine and 12.5 mg of sertraline, with a mass ratio of 1:12.5.
Embodiment 2
[0083] Example 2 Prepare reboxetine-sertraline compound tablets (1000 tablets) containing 32 mg reboxetine and 150 mg sertraline.
[0084] Formula: Reboxetine 32g
[0085] Sertraline 150g
[0086] Lactose 50g
[0087] Microcrystalline Cellulose 50g
[0088] Starch 10g
[0089] Sodium carboxymethyl starch 30g
[0091] Preparation method: as in the compound method of Example 1, each tablet contains 32 mg of reboxetine and 150 mg of sertraline in the prepared compound tablet, and its mass ratio is 16:75.
Embodiment 3
[0092] Example 3 Reboxetine-citalopram compound tablets (1000 tablets) containing 4 mg reboxetine and 20 mg citalopram were prepared.
[0093] Formula: Reboxetine 4g
[0094] Citalopram 20g
[0095] Lactose 50g
[0096] Microcrystalline Cellulose 50g
[0097] Starch 10g
[0098] Sodium carboxymethyl starch 30g
[0100] Preparation method: As in the preparation method of Example 1, each of the prepared compound tablets contains 4 mg of reboxetine and 20 mg of citalopram, and the mass ratio is 1:5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com