Propylamine derivative and its application in preparing tomocetin
A technology of atomoxetine and its derivatives, which is applied in the field of propylamine derivatives and its application, can solve the problems of difficulty in raw material preparation, impact on product purity, high equipment requirements, etc., and achieve the effect of reasonable preparation route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
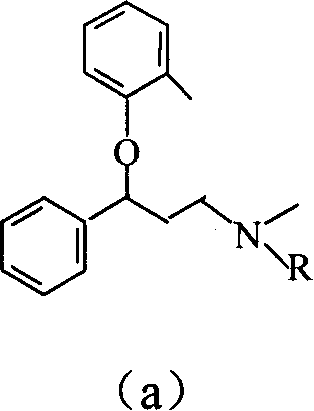
[0061] Preparation of N-methyl-N-benzyl-3-phenyl-3-(2-methylphenoxy)propylamine
[0062] (1) Preparation of N-methyl-N-benzyl-3-phenyl-3-carbonyl-propylamine hydrochloride:
[0063] Add 30 mL of concentrated hydrochloric acid dropwise to 36.2 g (0.30 moL) of N-methylbenzylamine until pH=2, and remove the solvent by rotary evaporation to obtain benzylamine hydrochloride. Dilute the obtained benzylamine hydrochloride, 11.2 g (0.37 moL) of paraformaldehyde and 30.0g (0.25moL) of acetophenone were added to a 250mL three-necked flask in turn, and 100mL of absolute ethanol was added as a solvent, and 5mL of concentrated hydrochloric acid was added, heated and stirred under reflux for 7h, cooled naturally, stirred at room temperature for 3h, and pumped After filtering, the filter cake was washed with ethanol, and dried to obtain 66.0 g of white solid, mp 192-194°C, HPLC content 99.9%, yield 92%.
[0064] 1 H-NMR(DMSO)δ: 2.70(s, 3H, N-CH 3 ), 3.40 (m, 2H, N-CH 2 ), 3.65 (m, 2H, CO...
Embodiment 2~5
[0072] The following compounds can be prepared in a manner similar to Example 1, specifically in Table 1
[0073] Table 1
[0074]
[0075] Application of N-methyl-N-benzyl-3-phenyl-3-(2-methylphenoxy)propylamine in the preparation of atomoxetine
Embodiment 7
[0077] (1) Preparation of N-methyl-N-ethoxycarbonyl-3-phenyl-3-(2-methylphenoxy)propylamine:
[0078] 34.5g (0.10moL) N-methyl-N-benzyl-3-phenyl-3-(2-methylphenoxy) propylamine, 4.1g (0.03moL) K 2 CO 3 , 16.2g (0.15mol) ClCO 2 Et was dissolved in 100mL toluene and heated to reflux for 3h. Heating was stopped and cooled naturally, the solvent was concentrated, and recrystallized from n-hexane to obtain 31.0 g of a yellow solid, mp: 79-81° C., HPLC content 99.6%. Yield 95%.
[0079] 1 H-NMR (CDCl 3 )δ: 1.15 (m, 3H, C-CH 3 ), 2.15 (m, 2H, CO-CH 2 ), 2.32(s, 3H, Ar-CH 3 ), 2.83 (s, 3H, N-CH 3 ), 3.45 (m, 2H, N-CH 2 ), 4.00 (m, 2H, CO 2 -CH 2 ), 5.15 (s, 1H, O-CH), 6.55-7.32 (m, 9H, Ar-H).
[0080] (2) Preparation of N-methyl-3-phenyl-3-(2-methylphenoxy)propylamine (tomoxetine):
[0081] Add 32.7g (0.10mol) of N-methyl-N-ethoxycarbonyl-3-phenyl-3-(2-methylphenoxy)propylamine, 22.4g (0.4mol) of KOH, and 200mL of n-butanol to In a 500mL three-neck flask, heat to reflux...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com