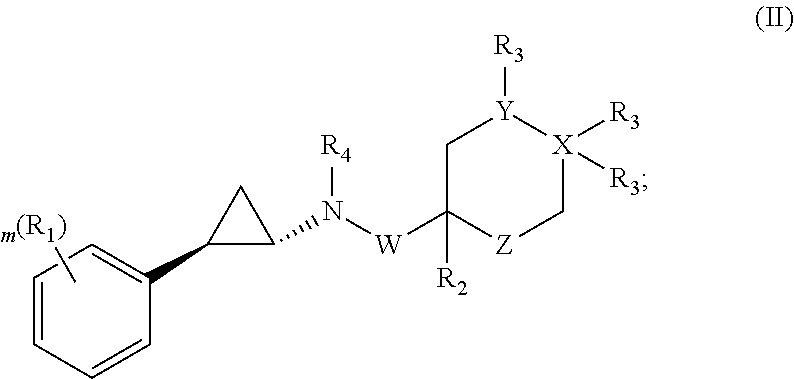
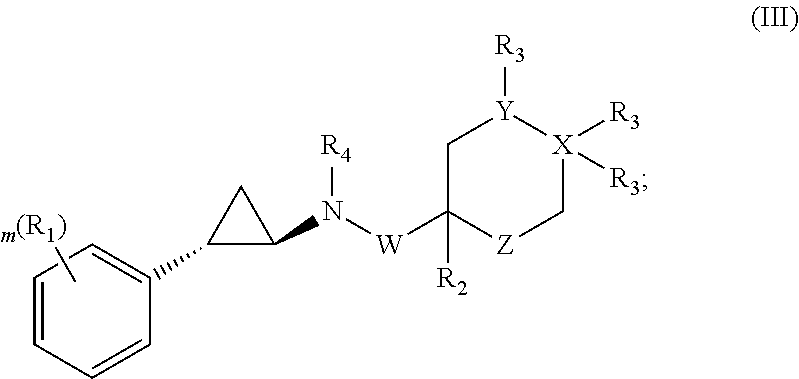
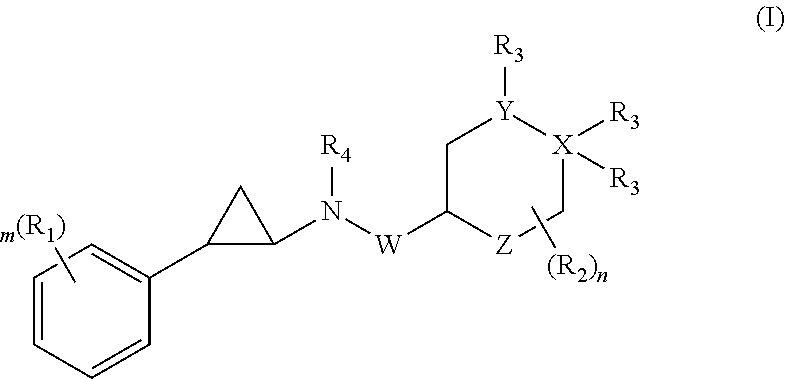
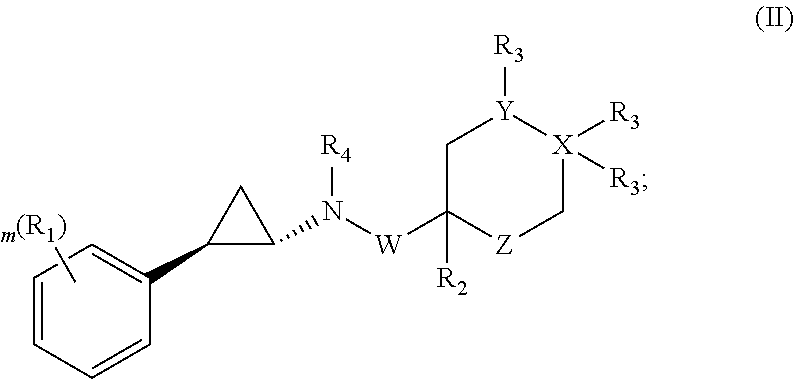
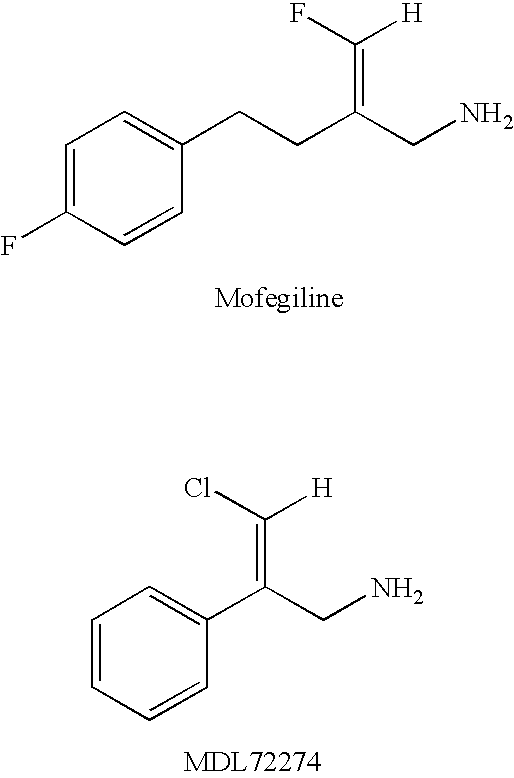
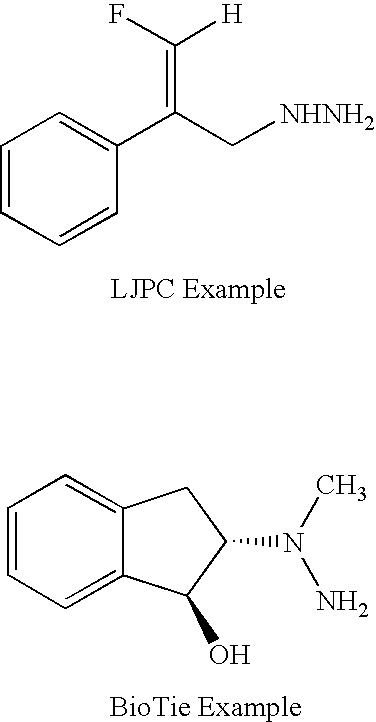
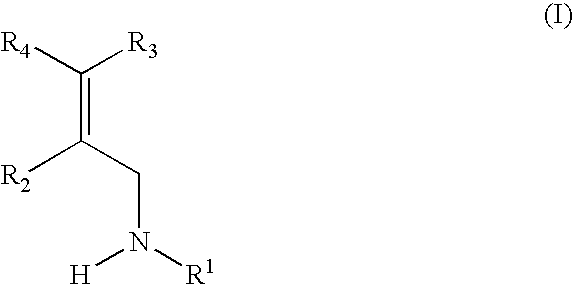
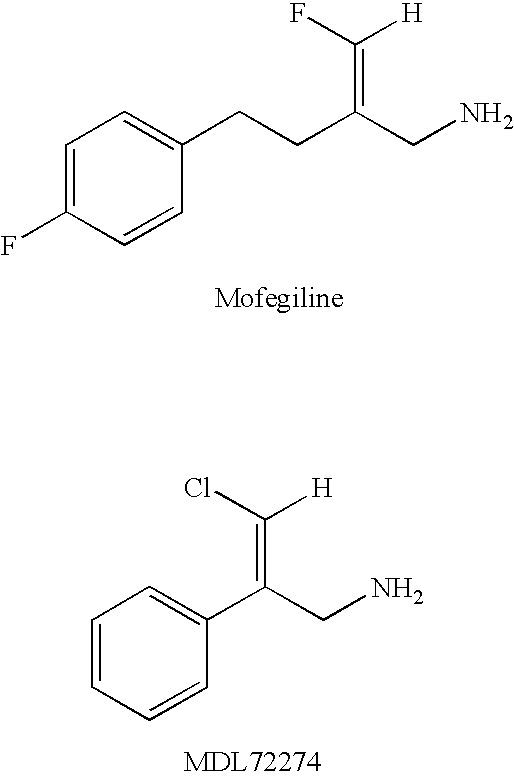
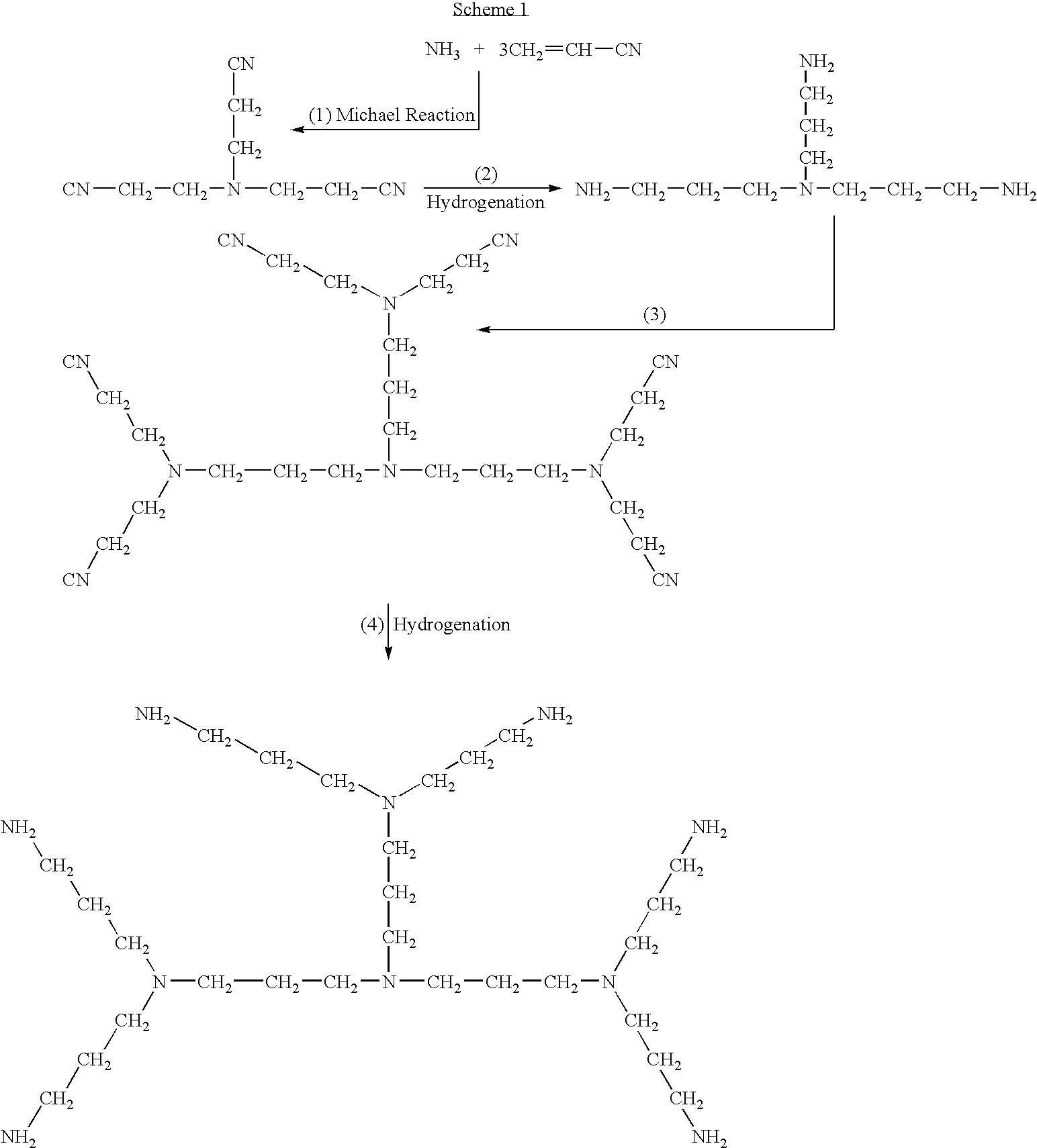
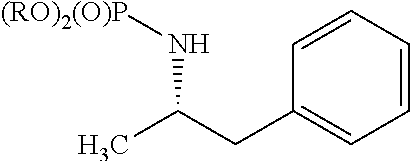
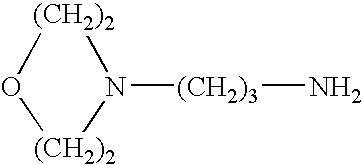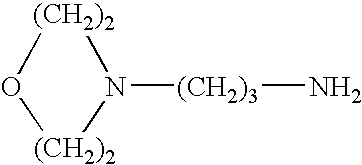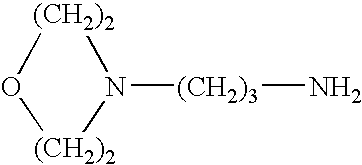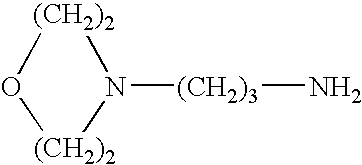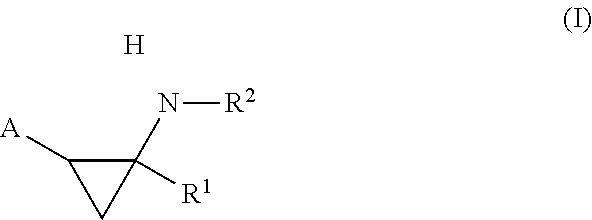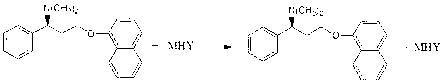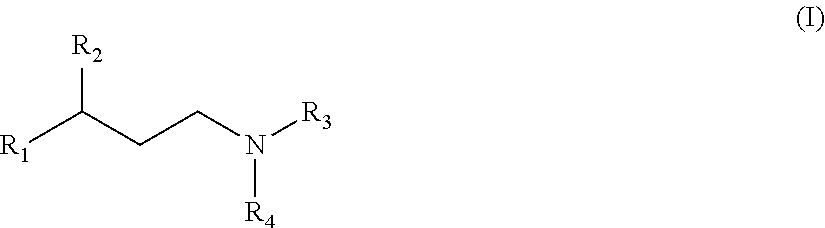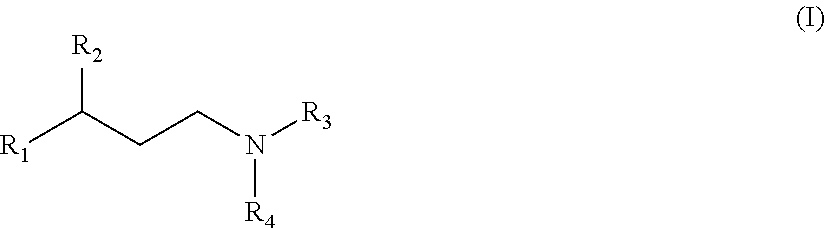Patents
Literature
47 results about "PROPYLAMINE DERIVATIVES" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
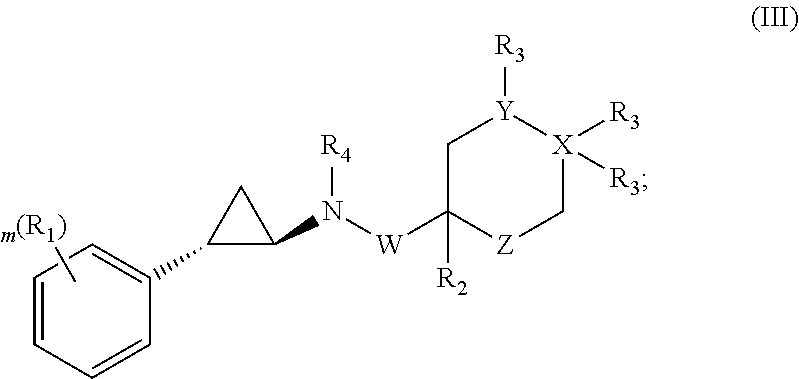
Phenylcyclopropylamine derivatives and their medical use
ActiveUS20120004262A1Avoids deleterious side-effectsLong half-lifeBiocideCompound screeningDiseaseMedicine
The present invention relates to phenylcyclopropylamine derivatives. In particular, pharmaceutical compositions comprising phenylcyclopropylamine derivatives are provided. The compounds of this invention can, inter alia, be used for the treatment and the prevention of cancer as well as neurodegenerative diseases or disorders.
Owner:ORYZON GENOMICS SA
Cyclopropylamines as LSD1 inhibitors
This invention relates to the use of cyclopropylamine derivatives for the modulation, notably the inhibition of the activity of Lysine-specific demethylase 1 (LSD1). Suitably, the present invention relates to the use of cyclopropylamines in the treatment of cancer.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Cyclopropylamines as lsd1 inhibitors
This invention relates to the use of cyclopropylamine derivatives for the modulation, notably the inhibition of the activity of Lysine-specific demethylase 1 (LSD1). Suitably, the present invention relates to the use of cyclopropylamines in the treatment of cancer.
Owner:GLAXOSMITHKLINE INTELLECTUAL PROPERTY (NO 2) LTD
Phenylcyclopropylamine derivatives and their medical use
ActiveUS8993808B2Avoids deleterious side-effectsLong half-lifeBiocideCompound screeningDiseaseMedicine
The present invention relates to phenylcyclopropylamine derivatives. In particular, pharmaceutical compositions comprising phenylcyclopropylamine derivatives are provided. The compounds of this invention can, inter alia, be used for the treatment and the prevention of cancer as well as neurodegenerative diseases or disorders.
Owner:ORYZON GENOMICS SA
Shale hydration inhibition agent(s) and method of use
A water-based drilling fluid and method of using same are presented in this disclosure, which fluid is used in drilling wells through a formation containing a shale that swells in the presence of water. The drilling fluid comprises an aqueous based continuous phase; a weighting material; and a shale hydration inhibition agent (SHIA) selected from the group consisting of propylamine derivatives, hydrogenated poly(propyleneimine) dendrimers (HPPID), and polyamine twin dendrimers (PTD). In some embodiments, the SHIA of this disclosure is not hydrolyzed at a temperature in the range of from about 100° F. to about 500° F. The drilling fluid may further comprise a fluid loss control agent, an encapsulating agent, other additives, and combinations thereof. A method of reducing shale swelling during wellbore drilling is also described. The method comprises circulating in the subterranean well a water-based drilling fluid comprising an aqueous based continuous phase, a weighting material, and a SHIA.
Owner:SHRIEVE CHEM PRODS
Haloallylamine inhibitors of SSAO/VAP-1 and uses therefor
Owner:PHARMAXIS LTD
Haloallylamine inhibitors of SSAO/VAP-1 and uses therefor
Owner:PHARMAXIS LTD
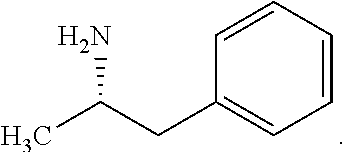
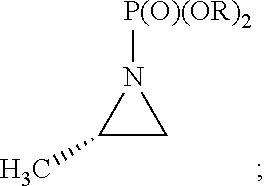
Synthesis of Chiral Amphetamine Derivatives by Stereospecific, Regioselective Cuprate Addition Reaction with Aziridine Phosphoramidate Compounds
Owner:CHEMAPOTHECA
Shale hydration inhibition agent(s) and method of use
A water-based drilling fluid and method of using same are presented in this disclosure, which fluid is used in drilling wells through a formation containing a shale that swells in the presence of water. The drilling fluid comprises an aqueous based continuous phase; a weighting material; and a shale hydration inhibition agent (SHIA) selected from the group consisting of propylamine derivatives, hydrogenated poly(propyleneimine) dendrimers (HPPID), and polyamine twin dendrimers (PTD). In some embodiments, the SHIA of this disclosure is not hydrolyzed at a temperature in the range of from about 100° F. to about 500° F. The drilling fluid may further comprise a fluid loss control agent, an encapsulating agent, other additives, and combinations thereof. A method of reducing shale swelling during wellbore drilling is also described. The method comprises circulating in the subterranean well a water-based drilling fluid comprising an aqueous based continuous phase, a weighting material, and a SHIA.
Owner:SHRIEVE CHEM PRODS
Synthesis of chiral amphetamine derivatives by stereospecific, regioselective cuprate addition reaction with aziridine phosphoramidate compounds
The invention includes processes for the synthesis of amphetamine, dexamphetamine, methamphetamine, derivatives of these, including their salts, and novel precursors and intermediates obtained thereby, by synthesizing aziridine phosphoramidate compounds in specified solvents at specified temperatures, and then converting to a novel aryl or aryl-alkyl phosphoramidate precursors using an organometallic compound such as a copper salt, where the novel aryl or aryl-alkyl phosphoramidate precursor is then easily converted to the target compounds using known reactions.
Owner:CHEMAPOTHECA
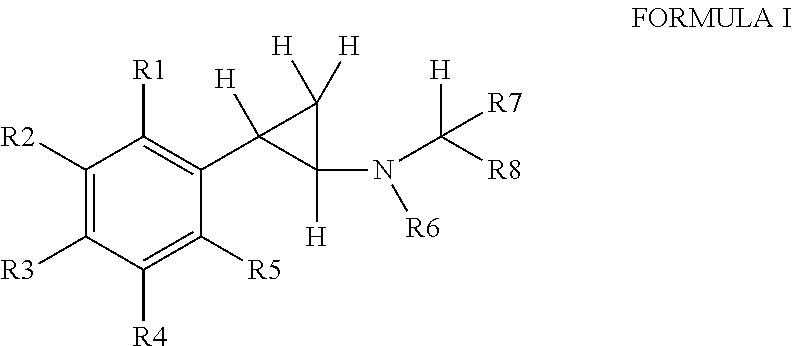
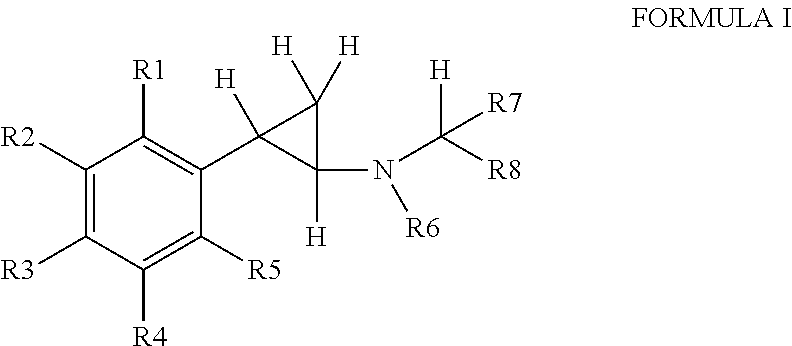
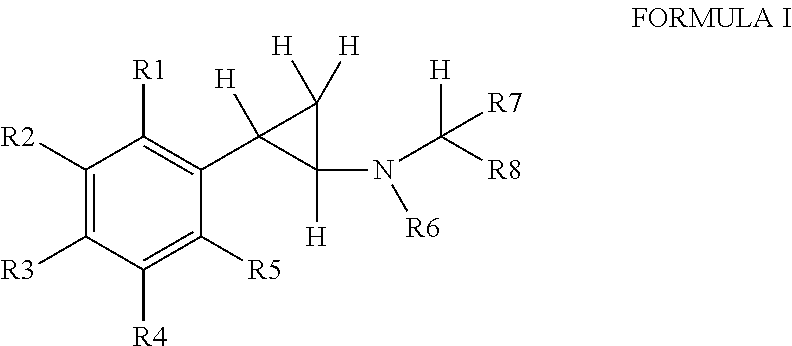
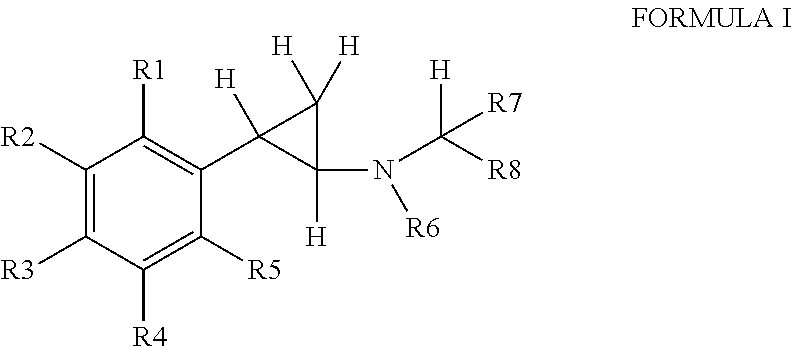
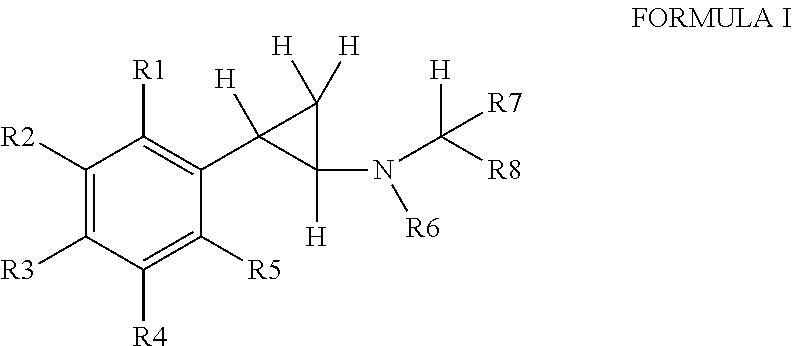
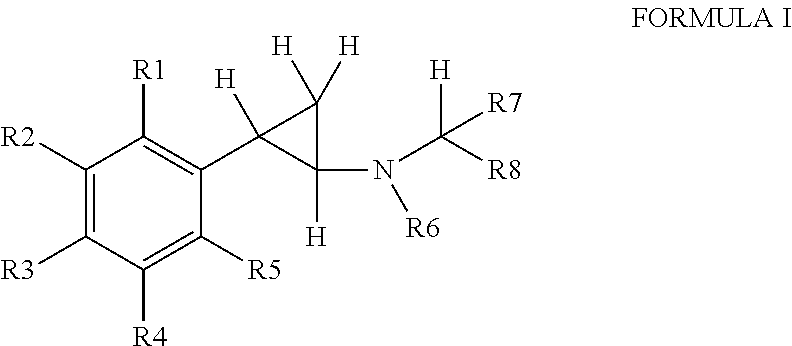
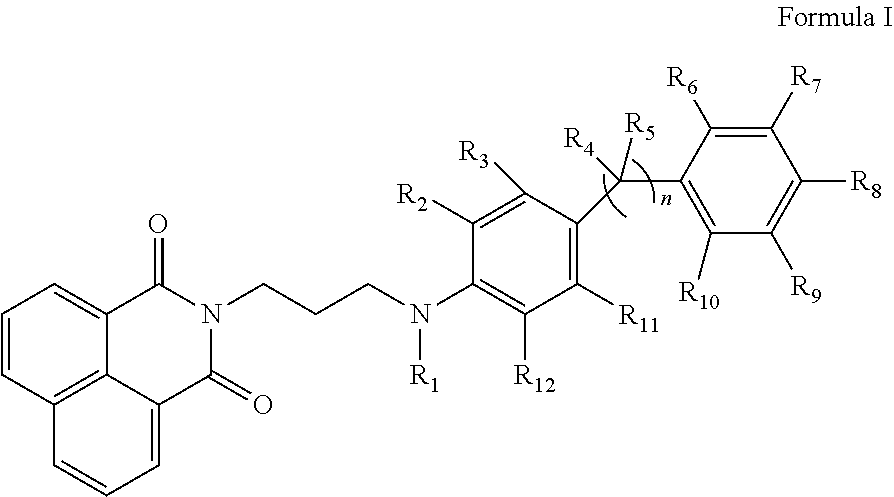
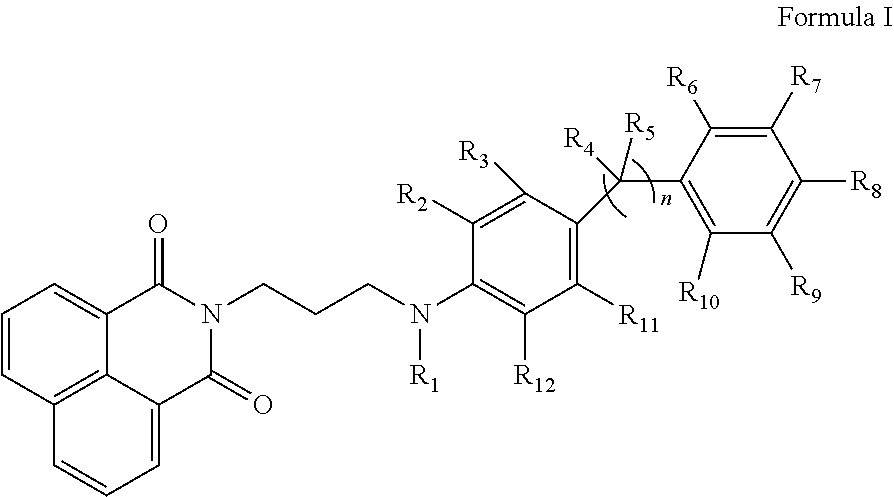
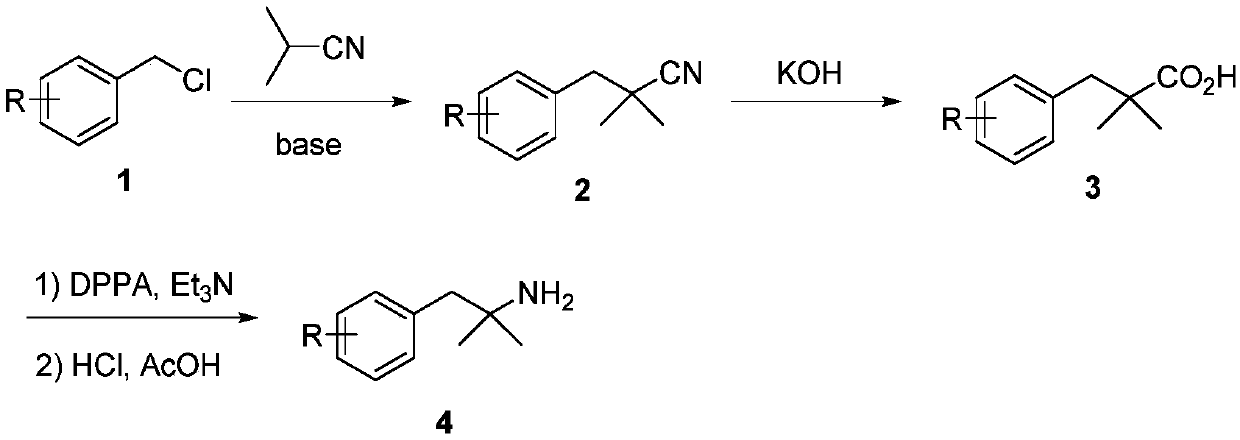
Substituted propylamine derivatives and methods of their use
The present invention is directed to substituted propylamine derivatives of formula I: or a pharmaceutically acceptable salt thereof, compositions containing these derivatives, and methods of their use for the prevention and treatment of conditions ameliorated by monoamine reuptake including, inter alia, vasomotor symptoms (VMS), sexual dysfunction, gastrointestinal and genitourinary disorders, chronic fatigue syndrome, fibromylagia syndrome, nervous system disorders, and combinations thereof, particularly those conditions selected from the group consisting of major depressive disorder, vasomotor symptoms, stress and urge urinary incontinence, fibromyalgia, pain, diabetic neuropathy, and combinations thereof.
Owner:WYETH LLC
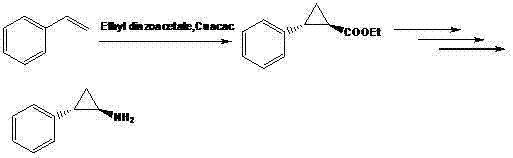
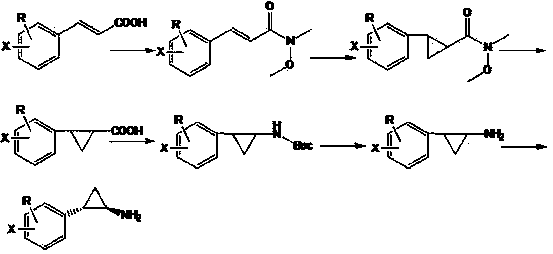
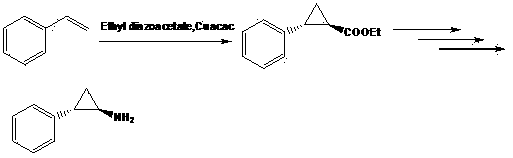
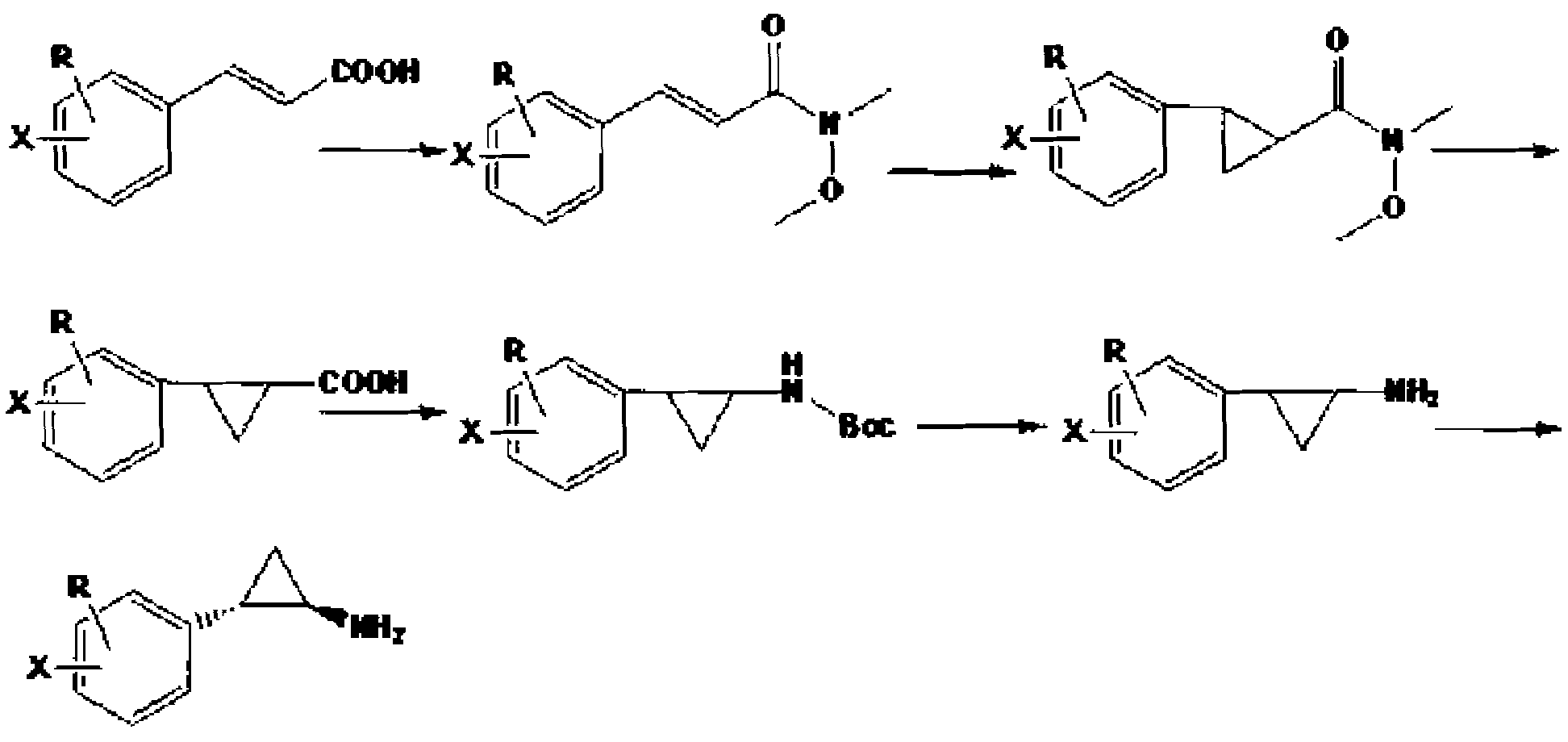
Preparation method of chiral aryl cyclopropylamine derivative
InactiveCN108083997AReduce usageShort reaction pathOrganic compound preparationCarboxylic acid esters preparationAcyl groupPropylamine
The invention provides a preparation method of a chiral aryl cyclopropylamine derivative. The chiral aryl cyclopropylamine derivative is prepared by using benzene halide or benzene polyhalide as the initial raw material and subjecting the benzene halide or benzene polyhalide to Friedel-Crafts reaction, asymmetric reduction reaction, cyclization reaction, acylation reaction, hydrolysis reaction andCurtius rearrangement reaction, wherein the benzene halide or benzene polyhalide is preferably o-difluorobenzene, 2-chlorofluorobenzene or fluorobenzene. The preparation method has the advantages that carbonyl asymmetric reduction and a acylation reagent are used to build a cyclopropyl ester structure, and the use of chiral auxiliaries is avoided; the reaction route of the method is shortened ascompared with a reaction route in the prior art, and reaction yield is increased; acyl azide rearrangement is used to prepare primary amine, and the method is simple to operate, high in yield and suitable for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
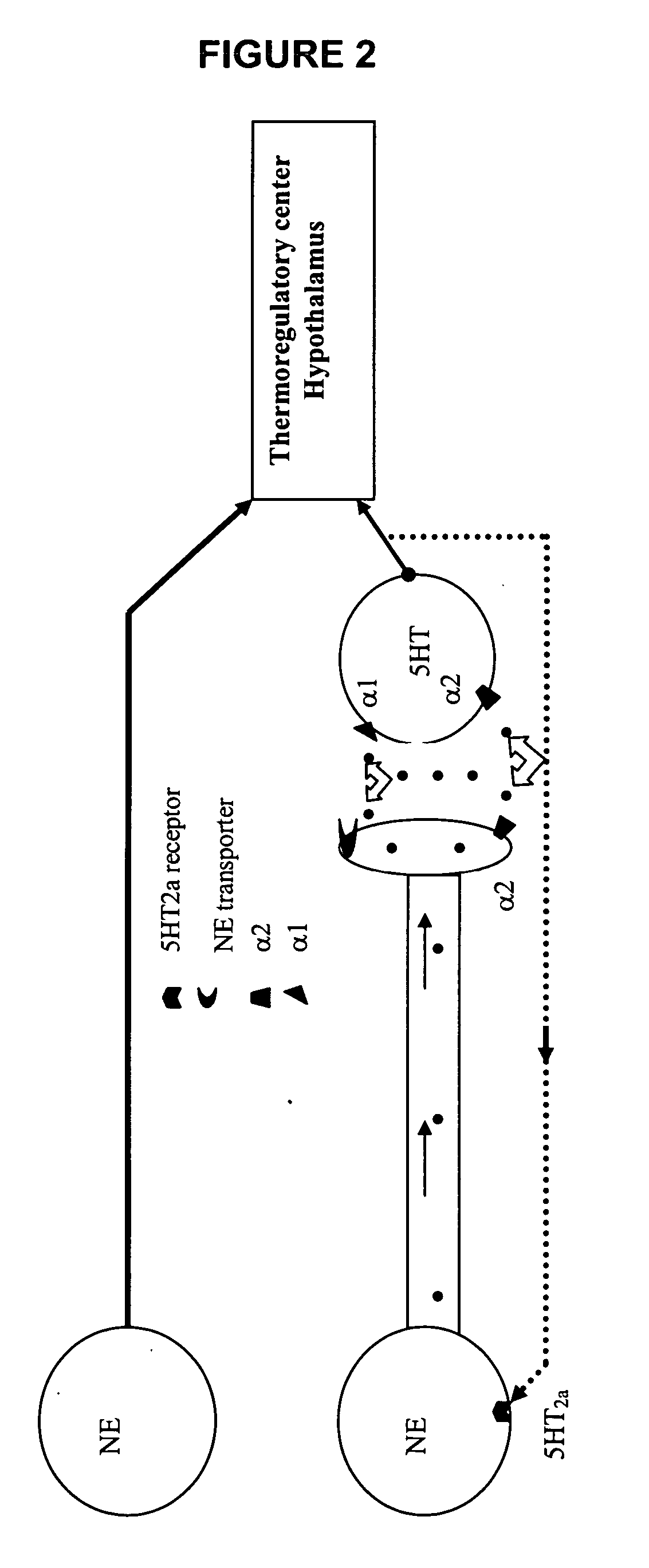
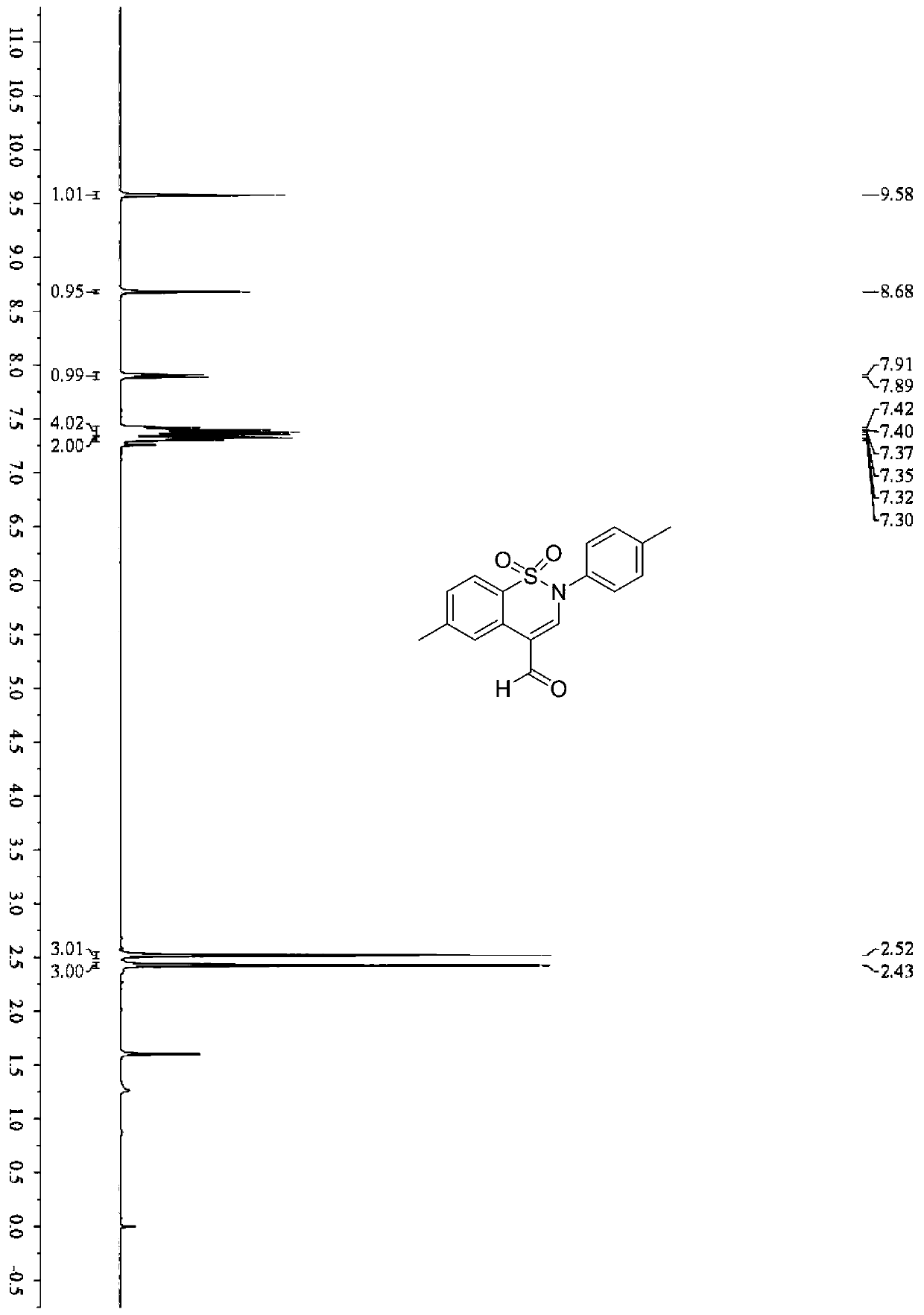
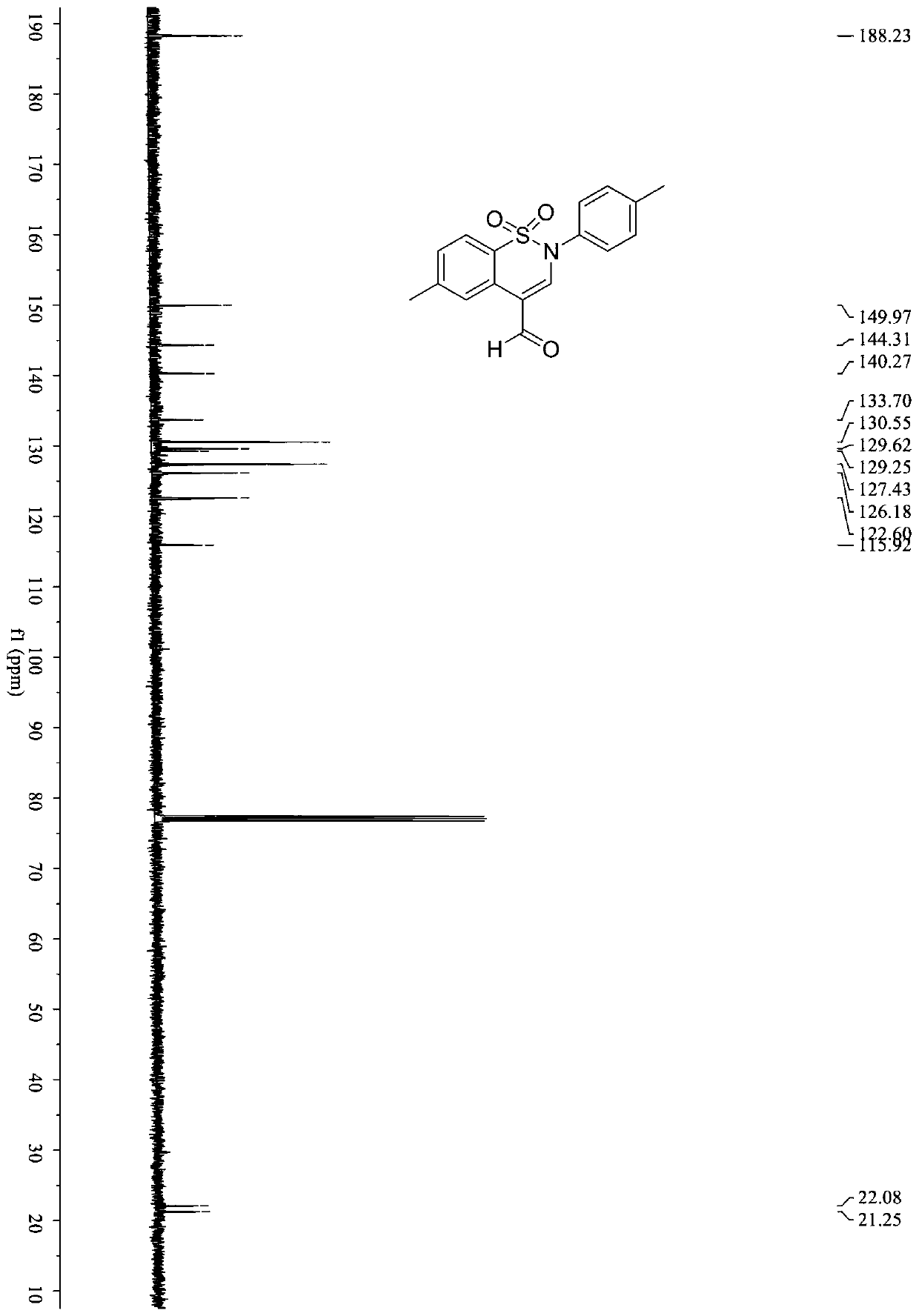
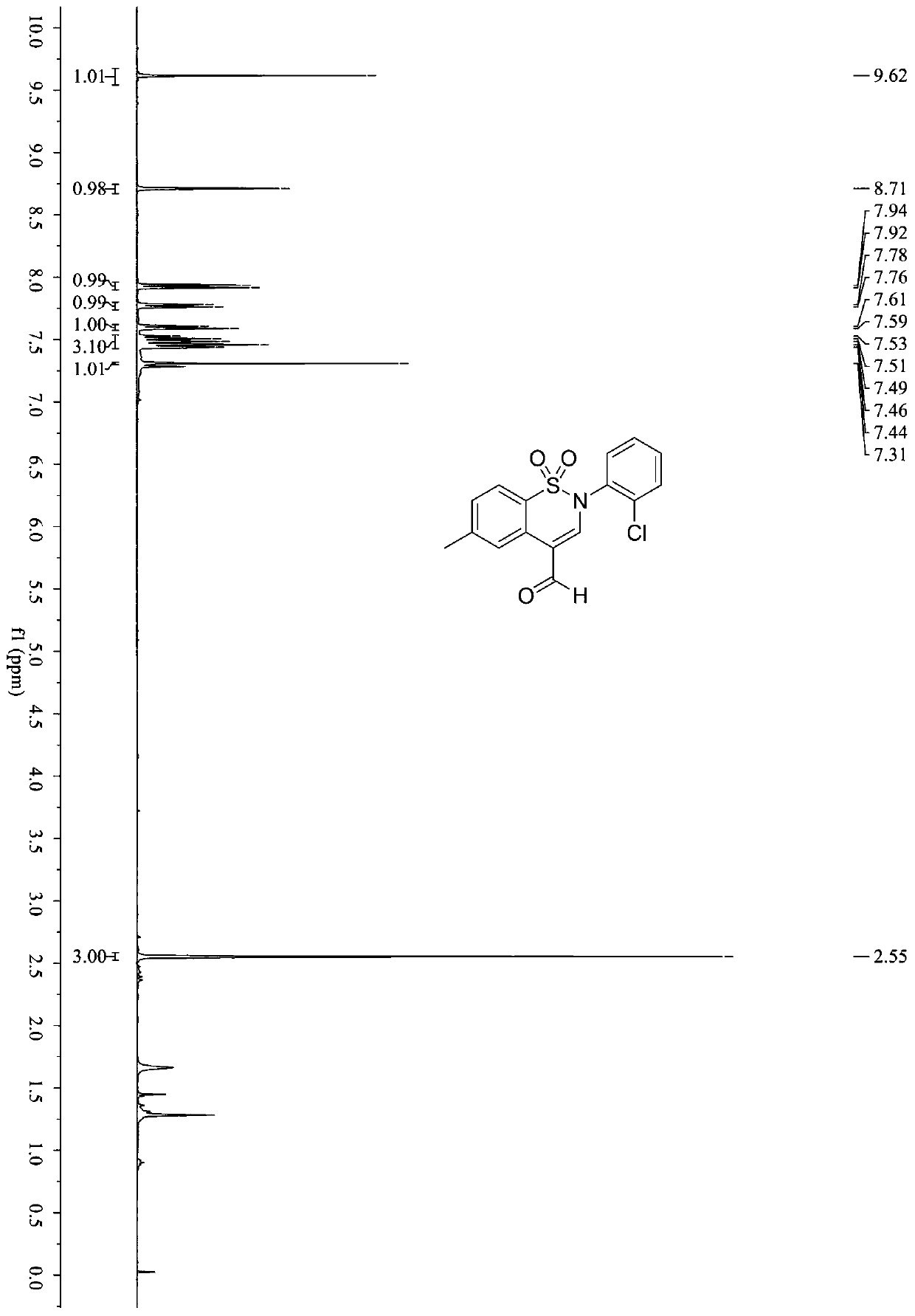
Synthetic method of benzothiazine formaldehyde derivative
The invention belongs to the technical field of benzothiazide derivatives, and discloses a synthetic method of a benzothiazide formaldehyde derivative. The synthesis method comprises the following steps: in the system of an organic solvent, an alkaline compound, a photocatalyst and an additive, carrying out a reaction on a benzenesulfonyl propargylamine derivative in an aerobic environment throughillumination to obtain a benzothiazine formaldehyde derivative, wherein the additive is more than one of diphenyl disulfide and thiophenol. According to the method, the benzothiazine compound is prepared by utilizing the carbon oxidation reaction of alkyne. The method is simple, convenient, efficient and high in regioselectivity, the used raw materials are simple and easy to obtain, no extra organic oxidizing agent needs to be added, oxygen in the air is used as an oxygen source and is environmentally friendly, cheap and easy to obtain. In addition, the whole operation process is simple and feasible, steps are simple and products are easy to purify.
Owner:SOUTH CHINA UNIV OF TECH
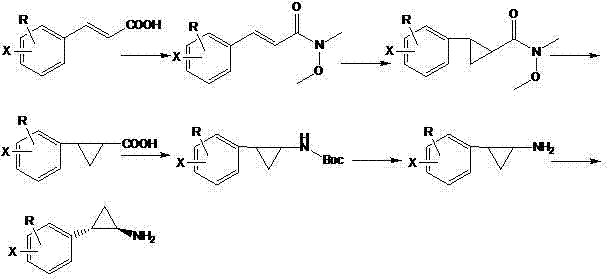
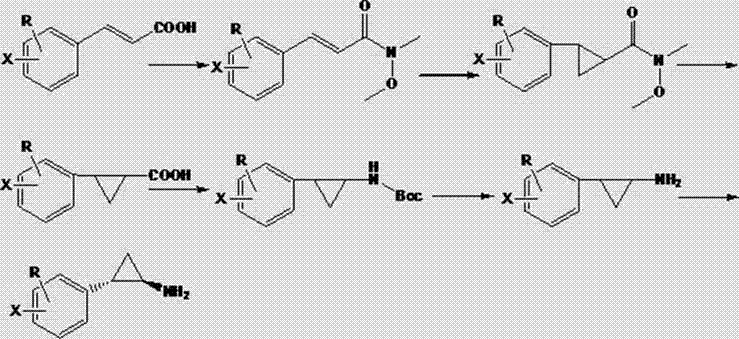
Chemical synthesis method of (1R, 2S)-2-aryl cyclopropylamine derivative
InactiveCN102863341AImprove e.e. valueAvoid the risk of racemization inAmino compound purification/separationOrganic compound preparationChemical synthesisHydroxylamine
The invention relates to a chemical synthesis method of a (1R, 2S)-2-aryl cyclopropylamine derivative. 3-aryl crylic acid serves as a raw material, the raw material and N,O-dimethyl hydroxy amine hydrochloride are subjected reaction to prepare a corresponding amide intermediate, cyclization reaction is conducted, 2- aryl cyclopropylamine is obtained; and finally D-mandelic acid serves as a resolving agent to obtain the (1R, 2S)-2-aryl cyclopropylamine derivative. The method has the advantages of being high in yield and high in e.e. value.
Owner:NANTONG UNIVERSITY
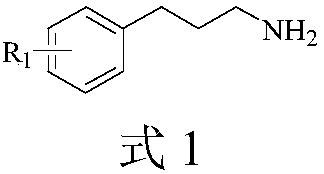
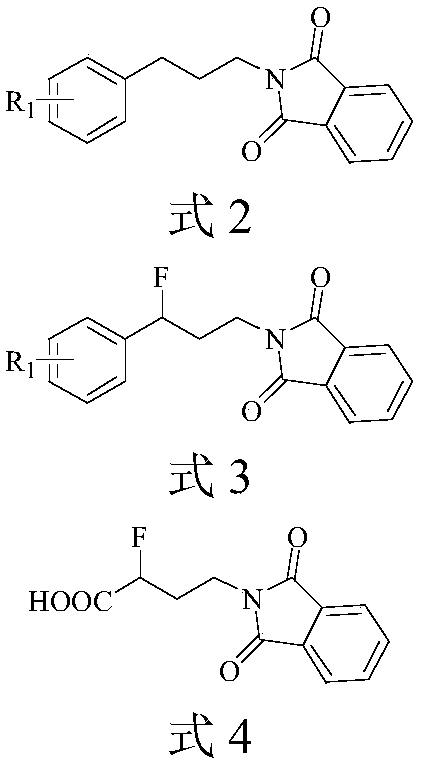
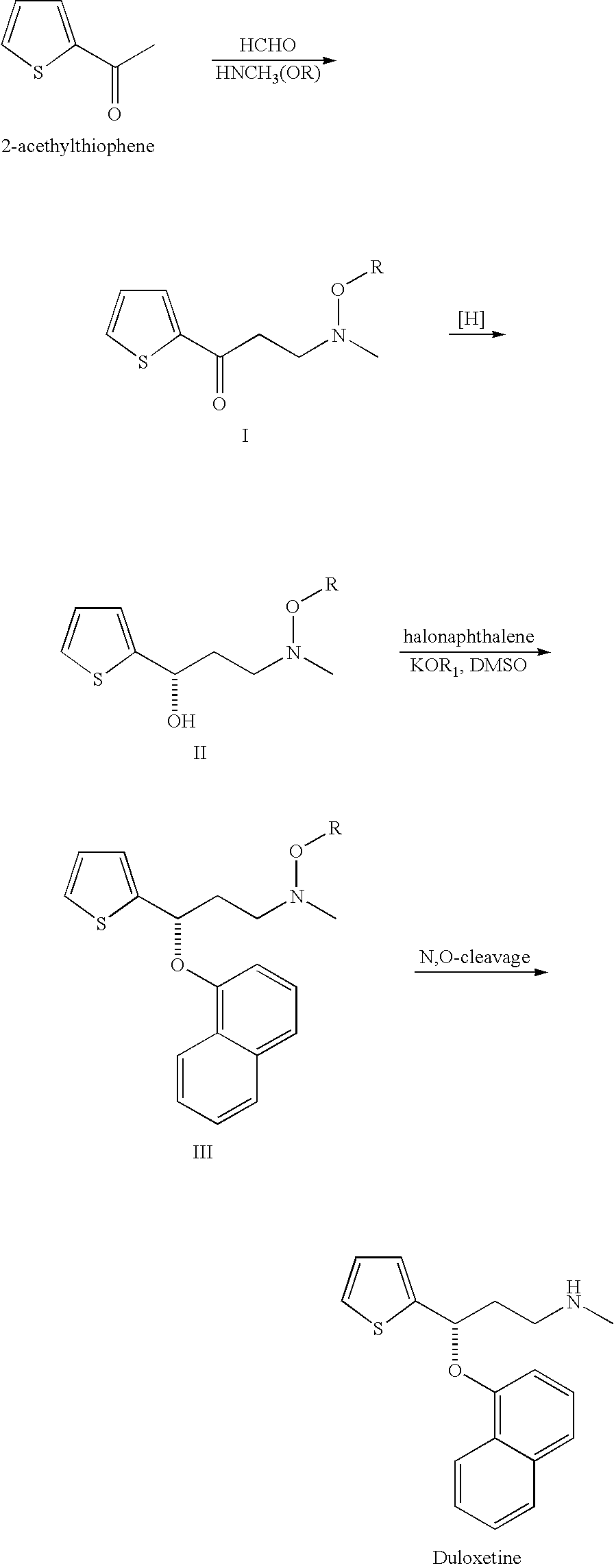
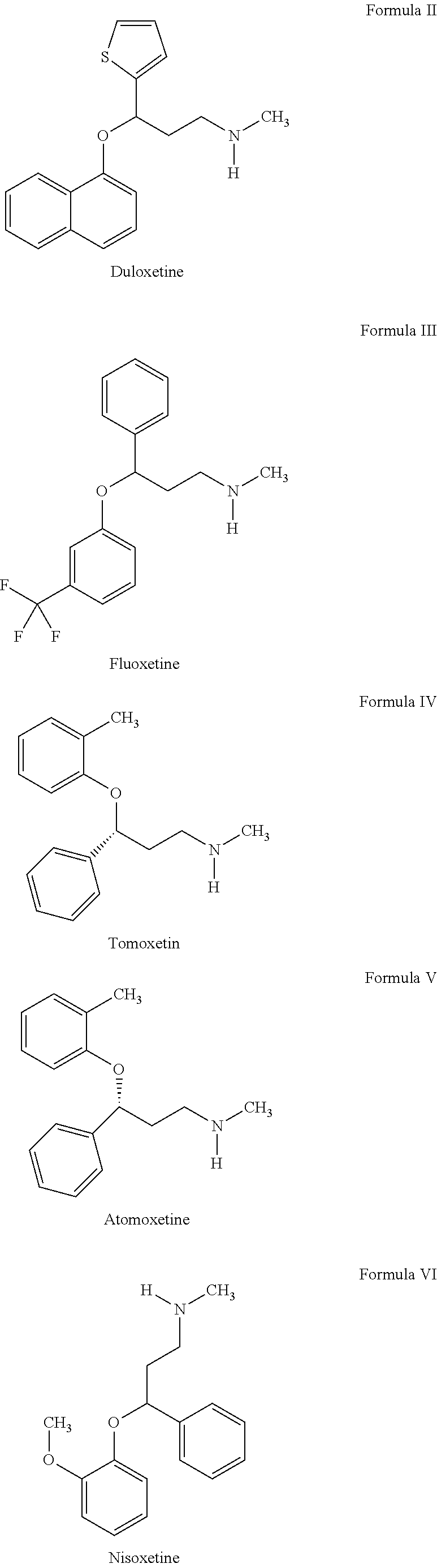
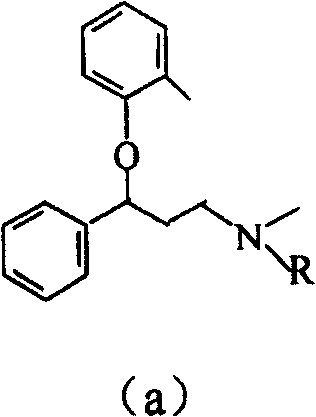
Propylamine derivative and its application in preparing tomocetin
InactiveCN1948277AThe preparation route is reasonable and feasibleSatisfactory yieldOrganic compound preparationAmino-hyroxy compound preparationState of artChemical structure
The present invention relates to a propylamine derivative and its application in preparation of tomoxetine (atomoxetine raceme). Said invention also provides its chemical structure formula, and the invented preparation method is simple, and is suitable for industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
Chemical synthesis method of (1R, 2S)-2-aryl cyclopropylamine derivative
InactiveCN102863341BImprove e.e. valueAvoid the risk of racemization inAmino compound purification/separationOrganic compound preparationChemical synthesisHydroxylamine
The invention relates to a chemical synthesis method of a (1R, 2S)-2-aryl cyclopropylamine derivative. 3-aryl crylic acid serves as a raw material, the raw material and N,O-dimethyl hydroxy amine hydrochloride are subjected reaction to prepare a corresponding amide intermediate, cyclization reaction is conducted, 2- aryl cyclopropylamine is obtained; and finally D-mandelic acid serves as a resolving agent to obtain the (1R, 2S)-2-aryl cyclopropylamine derivative. The method has the advantages of being high in yield and high in e.e. value.
Owner:NANTONG UNIVERSITY
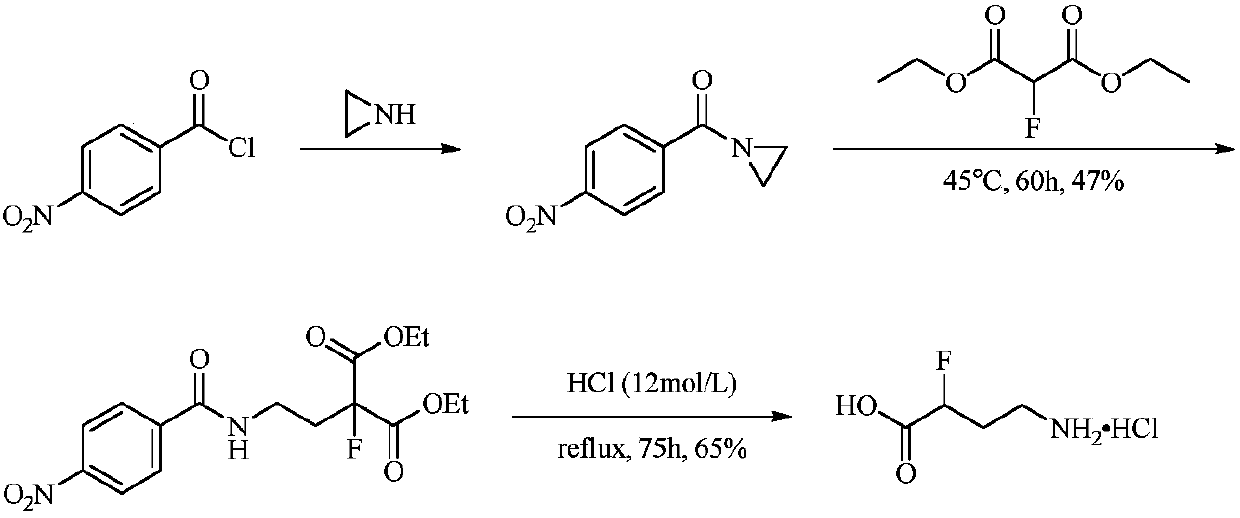
Synthesis method of (+/-)-alpha-fluoro-gamma-amino acid
ActiveCN107674015AGood choiceHigh yieldOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsPropylamine
The invention discloses a synthesis method of (+ / -)-alpha-fluoro-gamma-amino acid. The method comprises the steps of enabling 3-phenyl-1-propylamine or 3-phenyl-1-propylamine derivatives to be sequentially subjected to four reaction, i.e., amido protection, benzyl free radical fluorination, benzene ring oxidation and protecting group removal. The method avoids the use of a high-risk fluorine reagent DAST, and has the characteristics of being simple to operate, rapid in reaction, high in yield and the like.
Owner:JIANGXI NORMAL UNIV
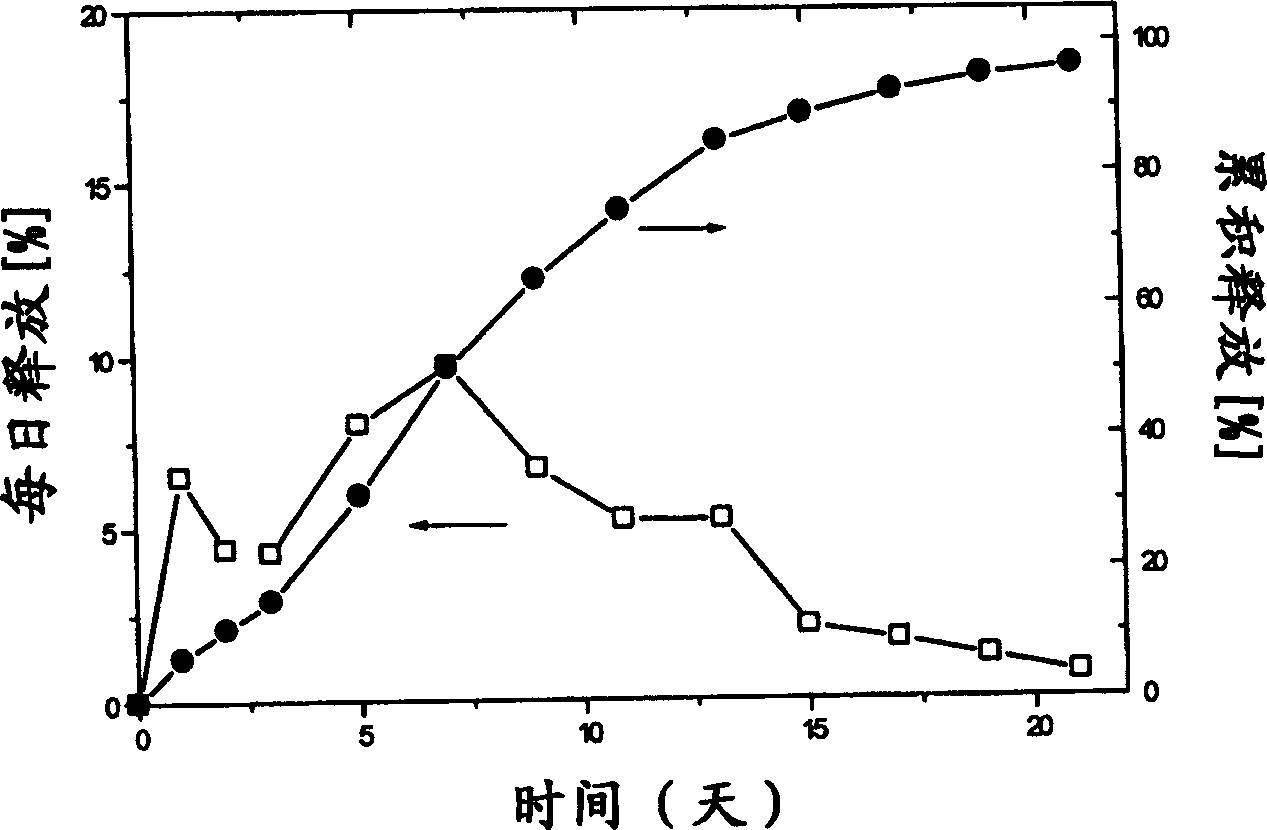
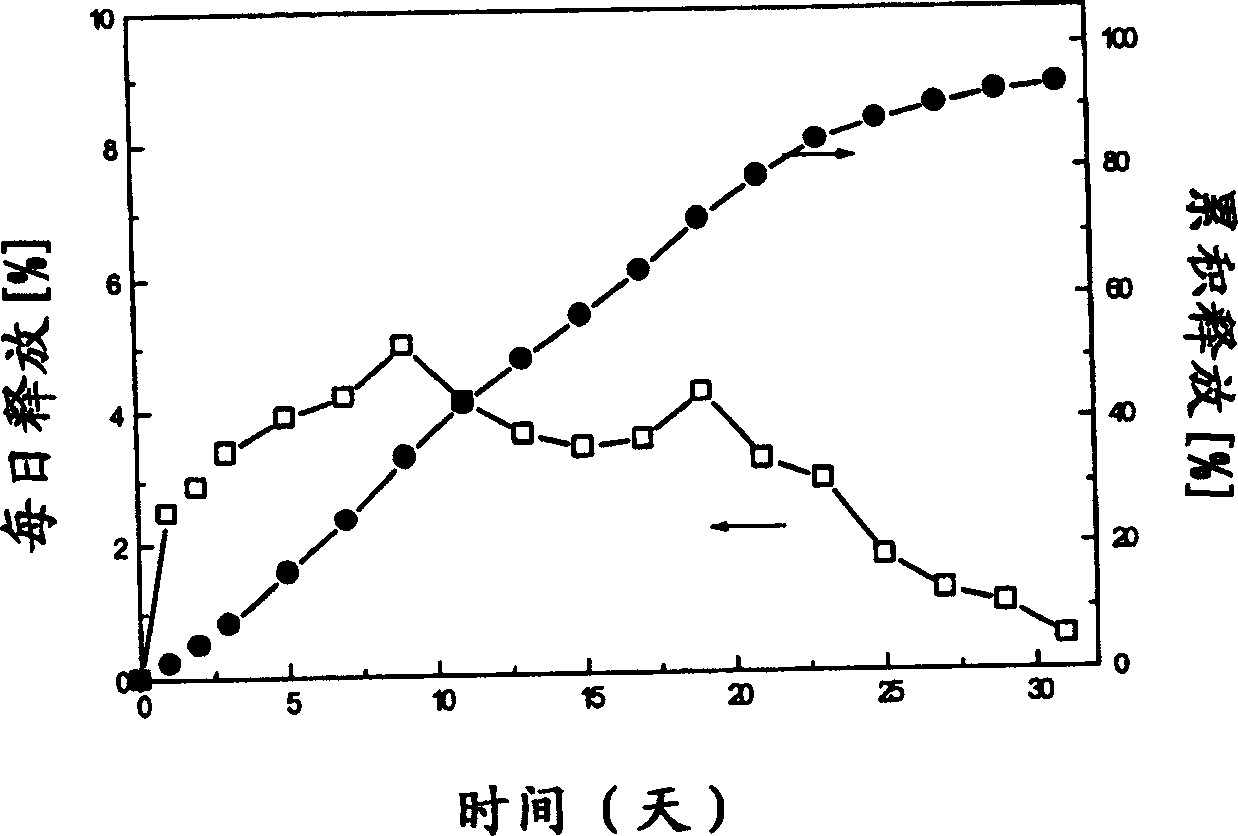
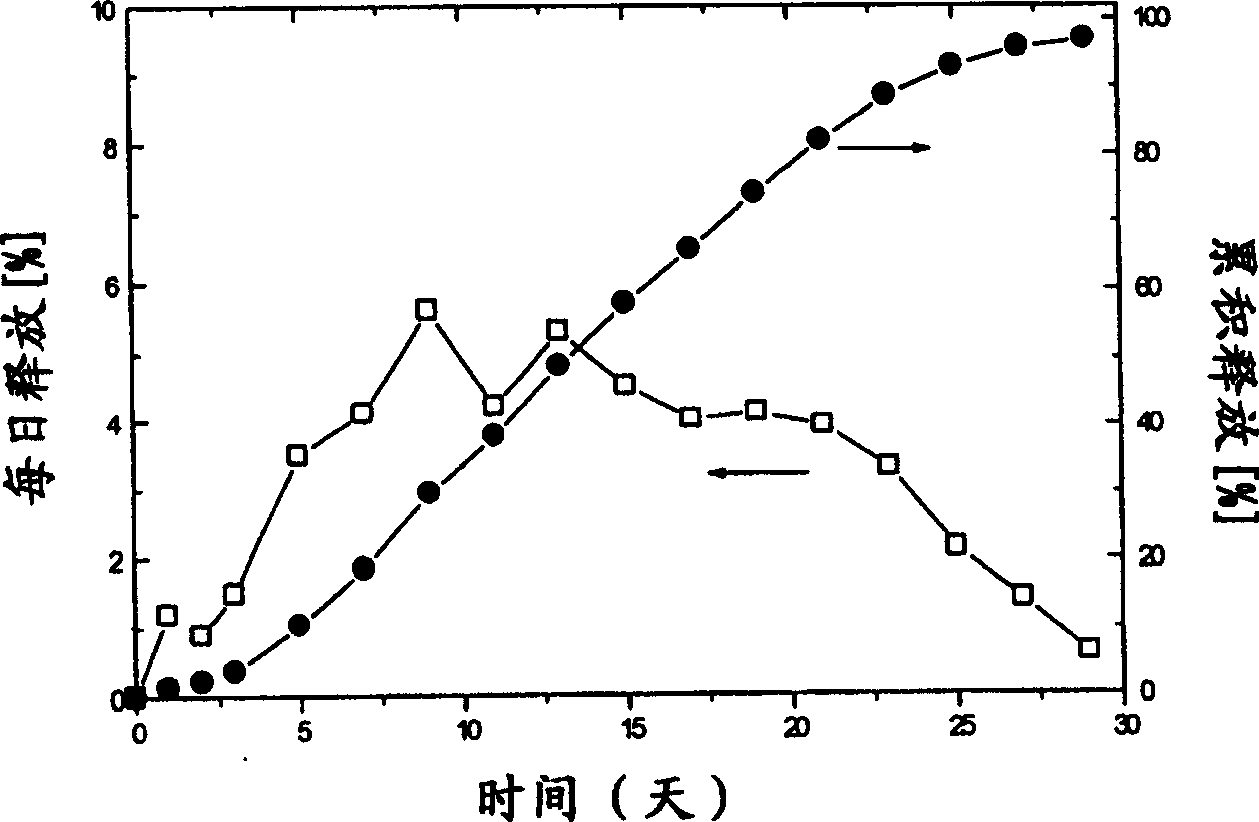
Slow release microsphere preparation of derivative of 3,3 ¿C diphenyl propylamine as receptor antagon of toadstool alkali in use for injection
InactiveCN1795845AAchieve long-term effectImprove the quality of lifeOrganic active ingredientsUrinary disorderDiseaseEnantiomer
A slowly-releasing microspherical medicine used for the injection to treat the diseases associated with the antagon of muscarinic receptor, the impulsive inoontinence and urination urgency or frequency is prepared from 3,3-diphenyl propylamine derivative, its optical enantiomer or cacemer, and the biodegradable medical high-molecular auxiliary. Its preparing process is also disclosed.
Owner:长春健欣生物医药科技开发有限公司
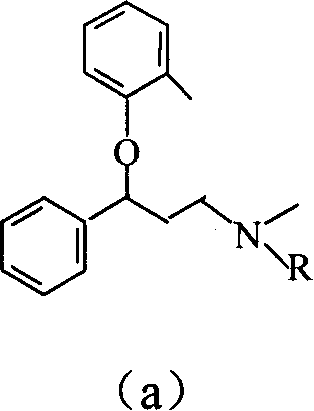
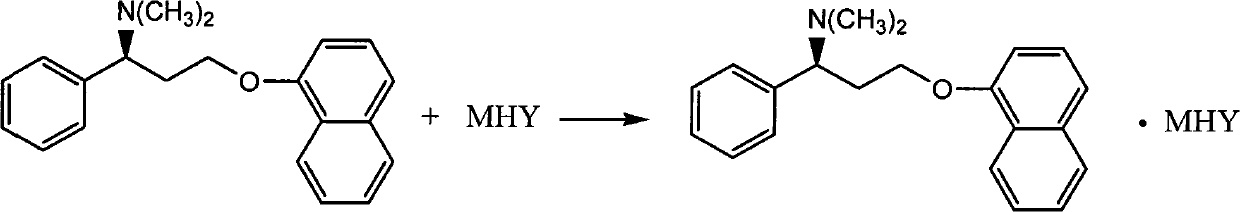
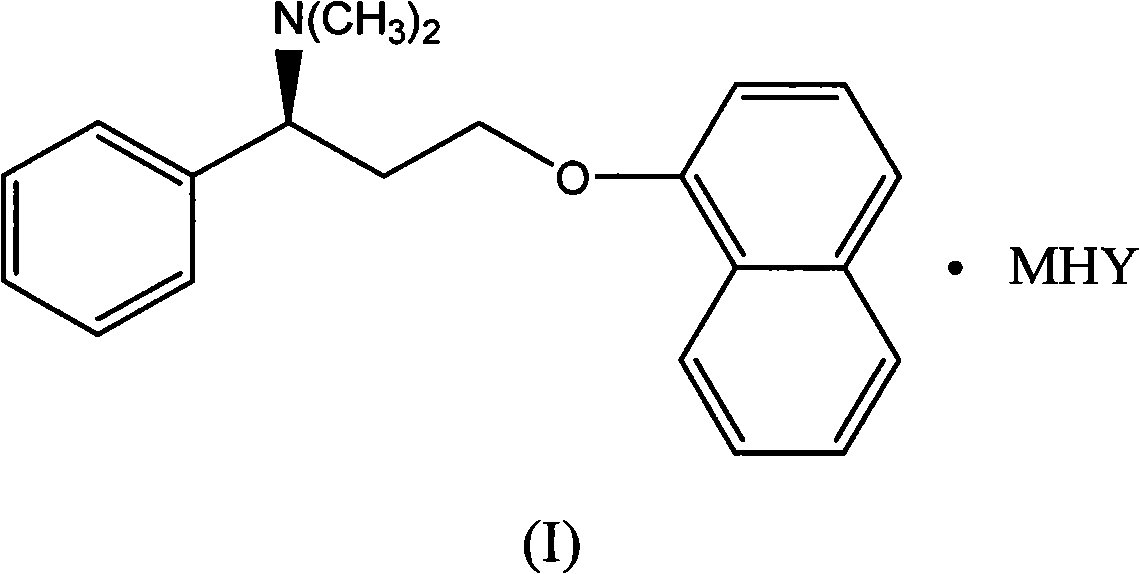
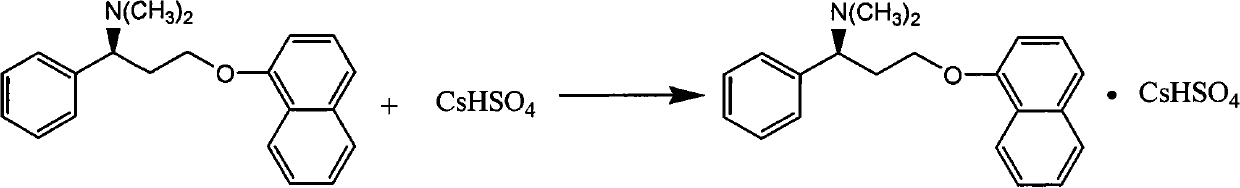
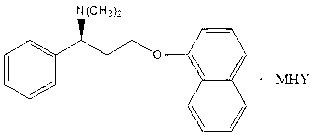
Naphthyloxy benzedrine derivatives and preparation method thereof
ActiveCN101955436AThe preparation method is reasonableSimple processOrganic compound preparationSexual disorderSexual impotenceSulfate radicals
The invention provides naphthyloxy benzedrine derivatives, which are prepared by subsequently reacting naphthyloxy benzedrine with acid and alkali metals or ammonium (including ammonia) compounds or amino acid or alkamine or directly reacting with acid salt; when the acid or acid salt is a sulfate radical, a naphthyloxy benzedrine sulfate complex salt is obtained; and when the acid or the acid salt is a phosphate radical, a naphthyloxy benzedrine phosphate complex salt is obtained. The method has the advantages of rational design, stable process and high production feasibility. The naphthyloxy benzedrine derivatives provided by the method have the remarkable advantages of high bioavailability, high purity, small side effect and the like. The naphthyloxy benzedrine derivatives can be prepared into a preparation, and has rapid absorption and quick response after oral administration by feeding the naphthyloxy benzedrine into blood, so that the curative effect of treating premature ejaculation and erectile dysfunction of men is better achieved. The naphthyloxy benzedrine derivatives have the structural formula shown in the description.
Owner:HANGZHOU ADAMERCK PHARMLABS INC
Method for synthesizing propargylamine derivative with different substituent groups at alkyne terminal
InactiveCN105481699AImprove qualityHigh yieldSilicon organic compoundsAmino preparation from aminesPolymer scienceOrganic synthesis
The invention belongs to the technical field of organic synthesis and belongs to the method for synthesizing a propargylamine derivative with different substituent groups at the alkyne terminal. Mutual transformation of different propargylamine is realized through carbon-carbon single-bond activation, specifically, a compound terminal alkyne and propargyl substitute secondary amine to serve as raw materials under the catalysis system of rare earth, and the propargylamine derivative with various substituent groups at the alkyne terminal is prepared. According to the method, sources of raw materials are wide, the preparation is easy, the operation is simple and convenient, the selectivity is controllable, and the yield is high.
Owner:FUDAN UNIV
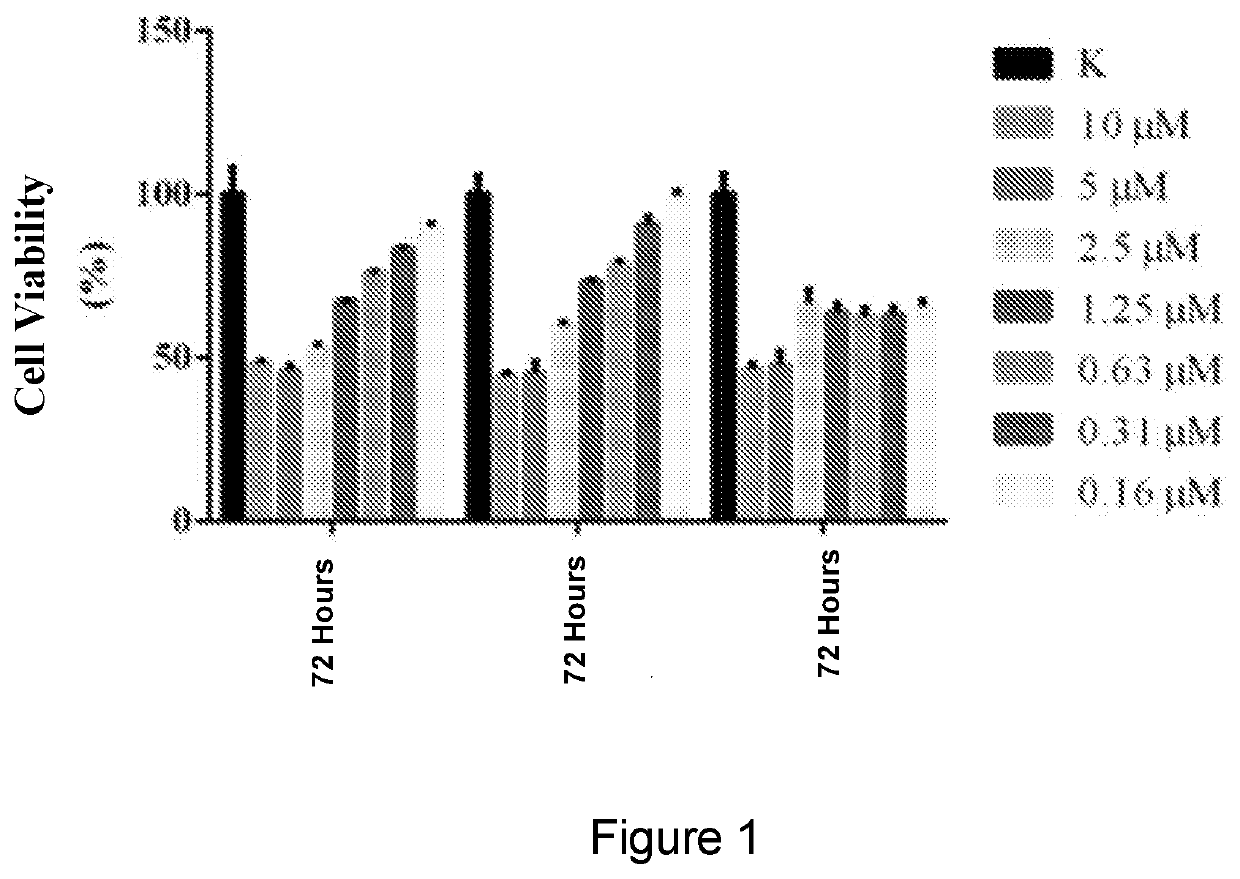
Naphthalimide derivatives as Anti-parasitic agents for the treatment of leishmaniasis as well as viral, bacterial and neoplastic diseases
Disclosed are naphthalimide derivatives and in particular e.g. N-aryl-substituted naphthalimidopropylamine derivatives (i.e. 2-[3-(amino)propyl]-1H-benz[de]isoquinoline-1,3(2H)-dione derivatives) such as e.g. such as e.g. (Formula I) or (Formula III) as anti-parasitic agents for the treatment of Leishmaniasis. The compounds could also be useful to treat viral, bacterial and / or neoplastic diseases. The description discloses exemplary synthesis as well as biological tests against Leishmania infantum parasites (e.g. pages 54 to 58; examples 1 to 7). Exemplary compounds are: (example 3)(example 4) 2-(3-((5-amino naphthalene-1-yl)amino)propyl)-1H-benzo[de]isoquinoline-1, 3(2H)-dione (example 5) 2-(3-((5-amino naphthalene-1-yl)amino)propyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (example 6).
Owner:T C ISTANBUL MEDIPOL UNIVERSITESI +1
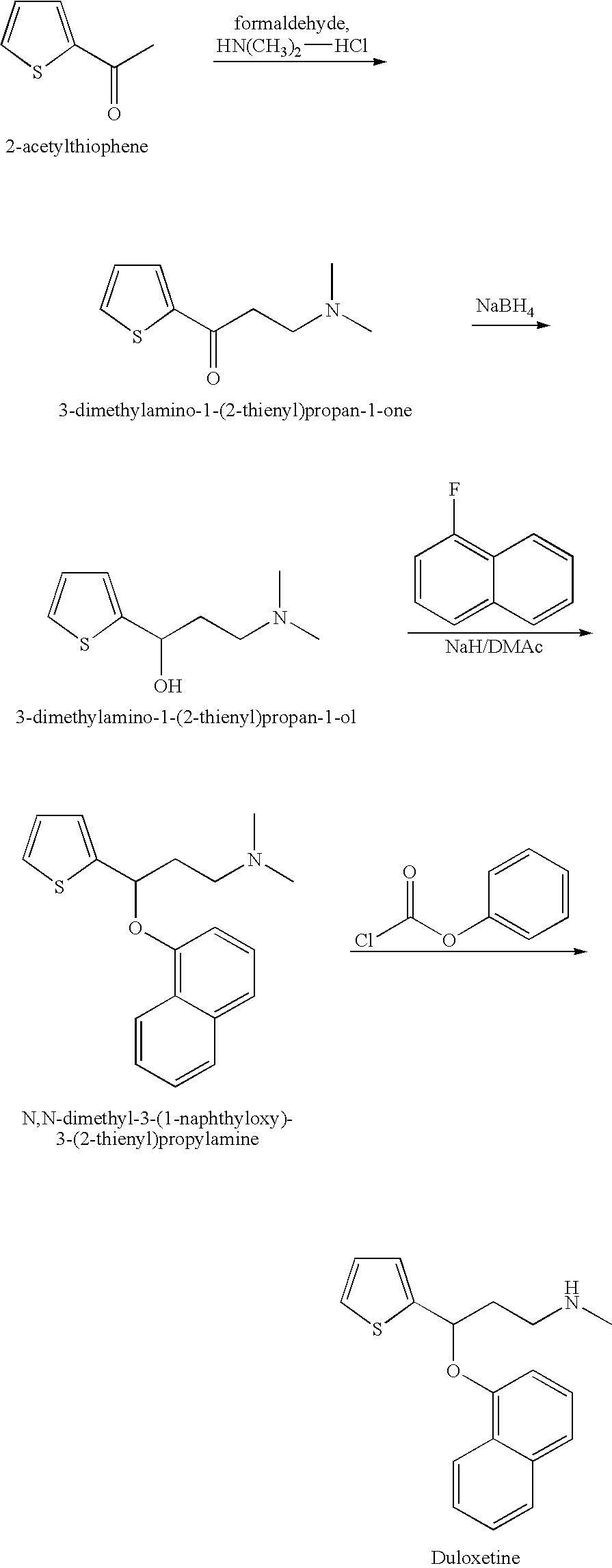
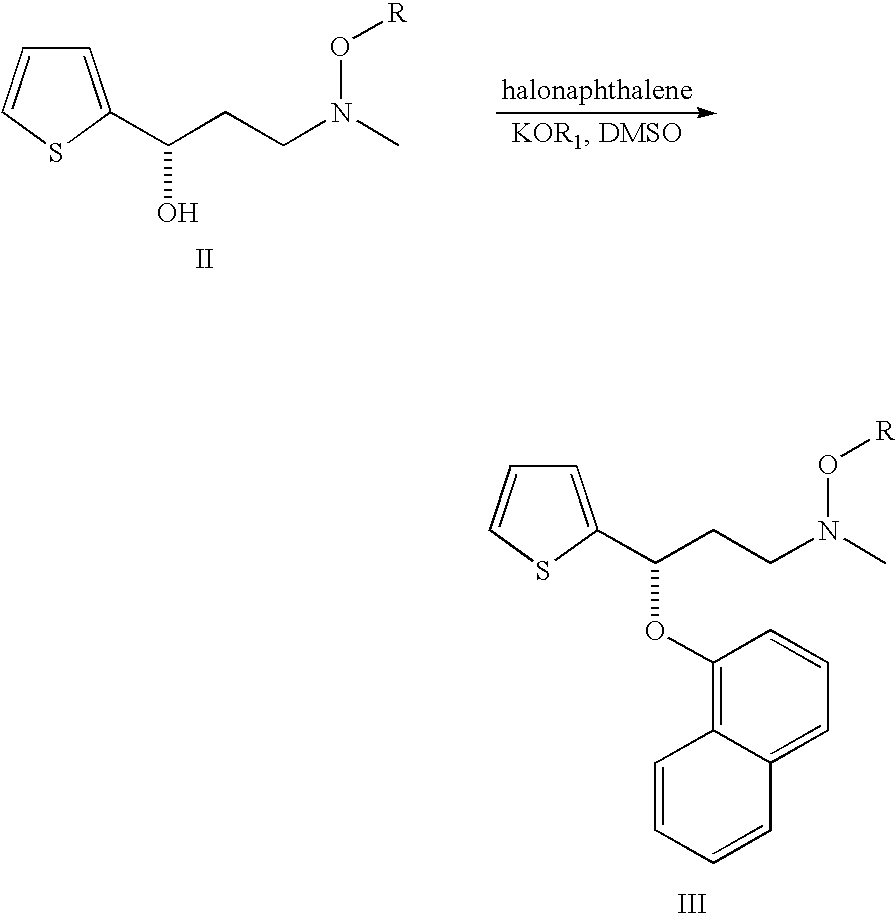
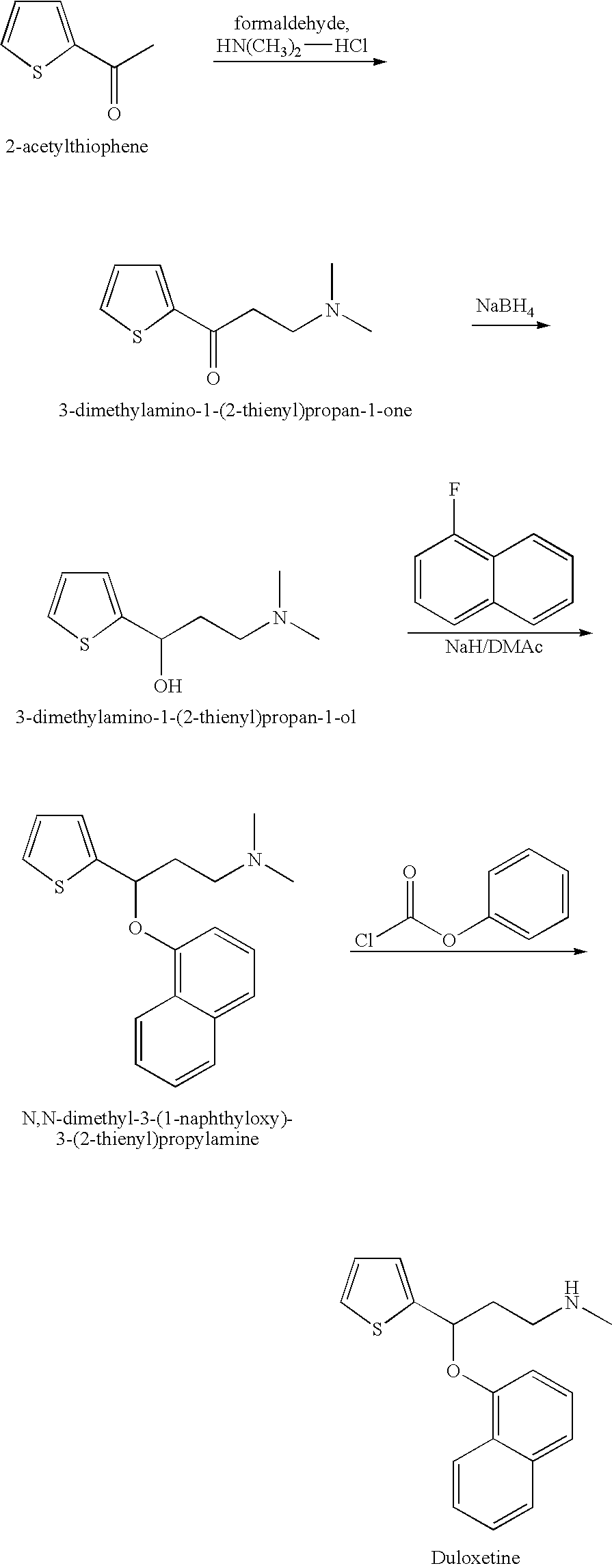
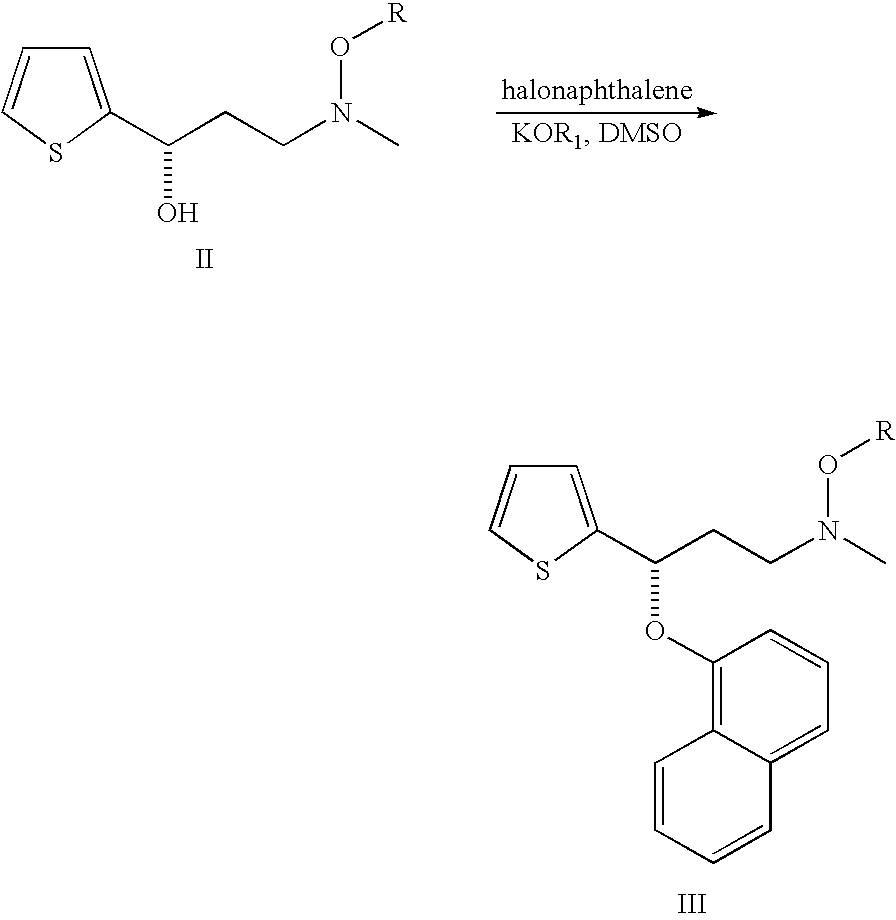
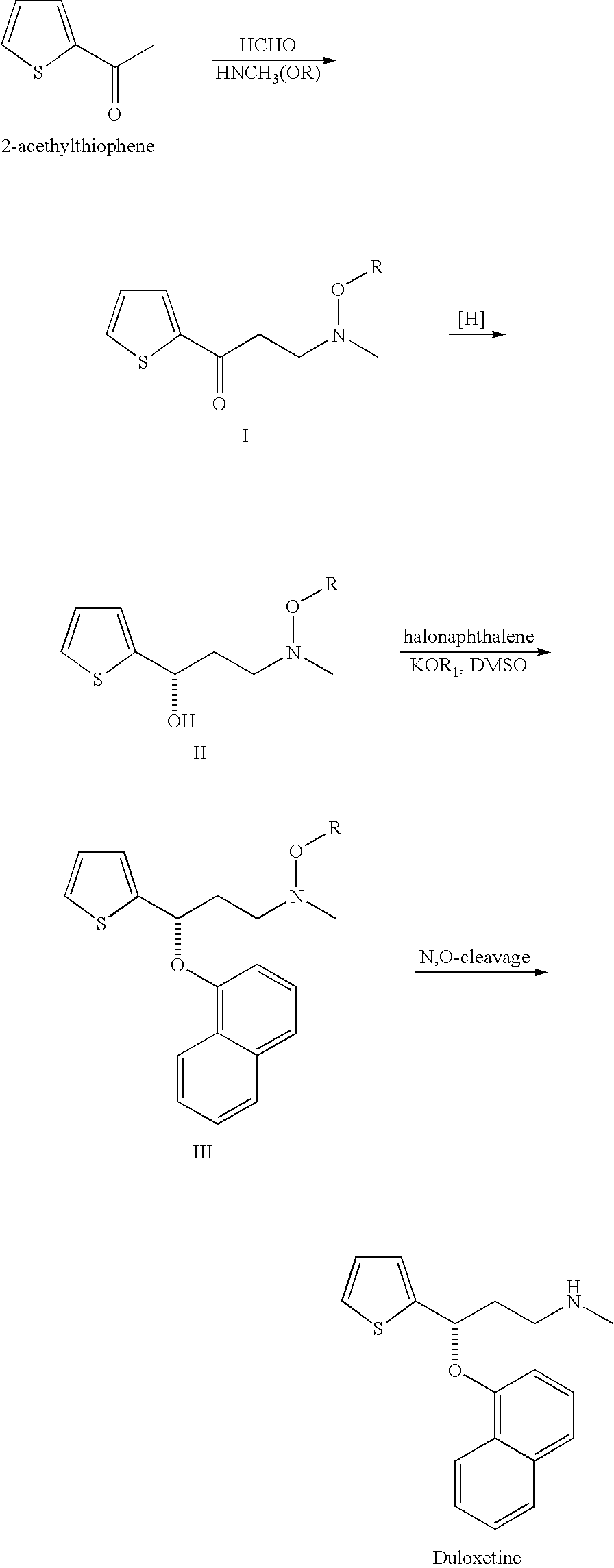
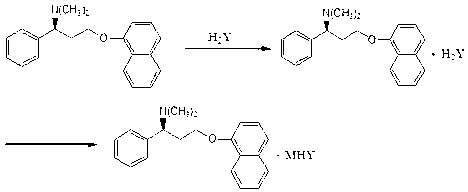
Process for preparing N-methyl-N-hydroxyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine derivative
The present invention provides a process for preparing a N-methyl-N-hydroxyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine compound. The present invention also provides a process for preparing (S)-(+)-N-methyl-3-methyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine with higher yield and low treatment cost, which includes the preparation of the N-methyl-N-hydroxyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine compound.
Owner:SCI PHARMTECH
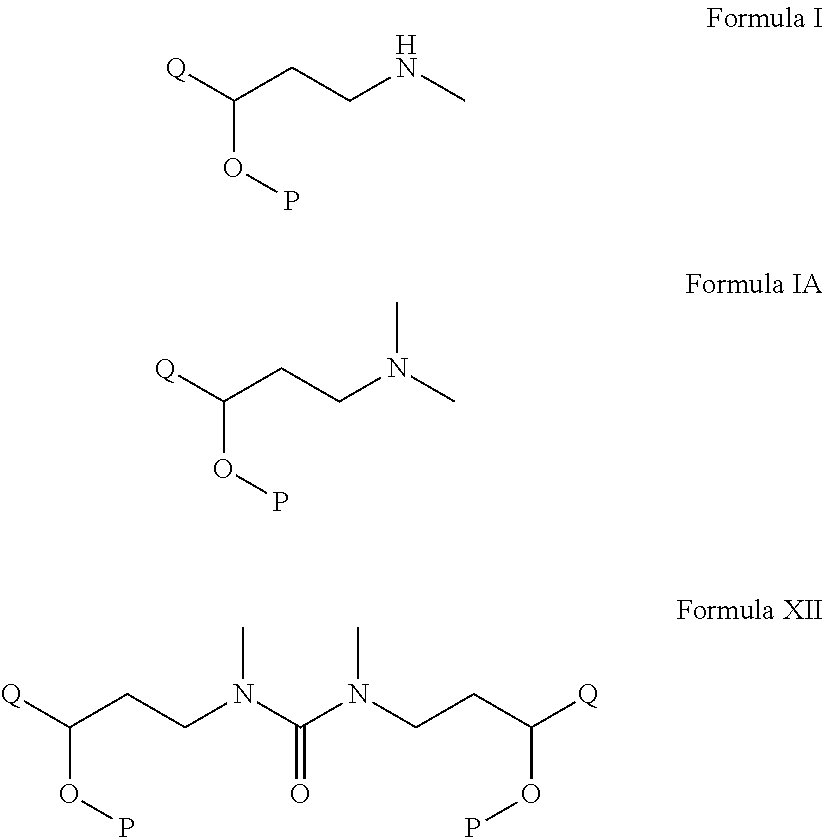
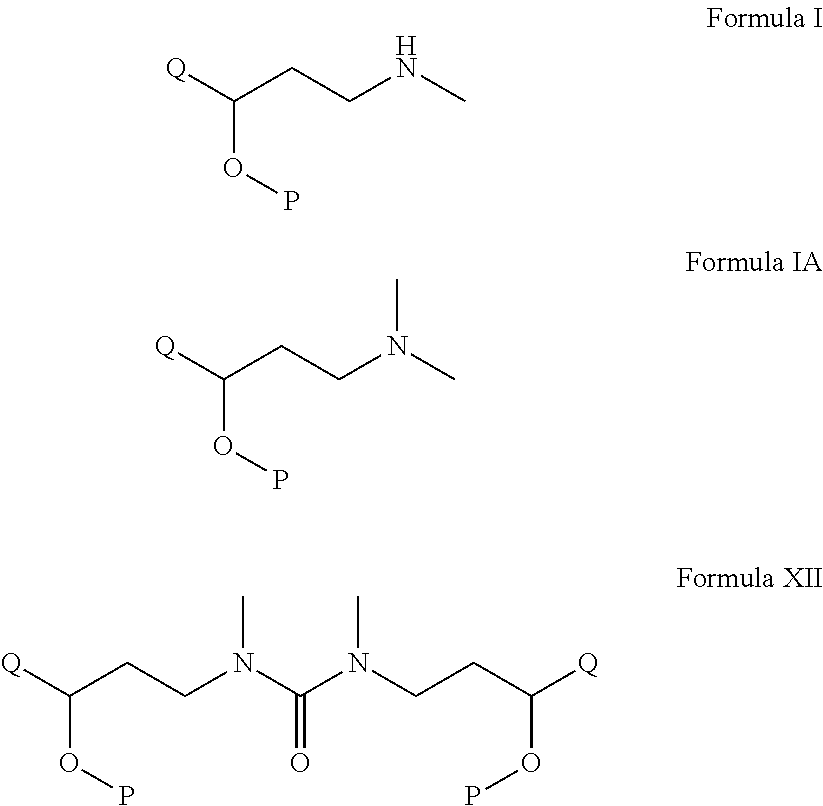
Process for the preparation of n-methyl-o-aryloxy propanamine derivatives and pharmaceutically acceptable salt thereof
A process for the preparation on N-methyl-aryloxy-propanamine derivatives of the formula I and salts thereof. The invention also relates to the preparation and use novel intermediate of the formula XII. The invention also relates to the process of further conversion of novel intermediate into N-methyl-aryloxy propanamine derivatives and salts thereof.Wherein Q and P independently represents substituted or unsubstituted aryl group such as phenyl, naphthyl, pyridine, furanyl, pyranyl thienyl, and the like optionally substituted aryl by a halogen, a straight chain or branched alkyl group containing 1 to 6 carbon atoms, —O-alkyl group containing straight chain or branched C1-C6 alkyl group, an alkoxy group containing a straight chain or branched alkyl group having 1 to 6 carbon atoms, which comprises demethylation of N,N-dimethyl analogues of compound of formula IA.
Owner:ARCH PHARMALABS LTD
Process for preparing n-methyl-n-hydroxyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine derivative
The present invention provides a process for preparing a N-methyl-N-hydroxyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine compound. The present invention also provides a process for preparing (S)-(+)-N-methyl-3-methyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine with higher yield and low treatment cost, which includes the preparation of the N-methyl-N-hydroxyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine compound.
Owner:SCI PHARMTECH
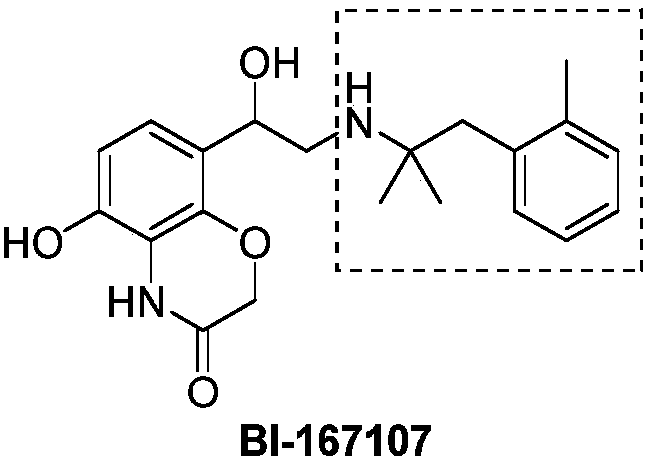
Cycloproplyamine derivatives useful as inhibitors of histone demethylases KDM1A
ActiveUS9944589B2Reduce weightOrganic active ingredientsCarbamic acid derivatives preparationHistone demethylationStereochemistry
Owner:INST EUROO DI ONCOLOGIA
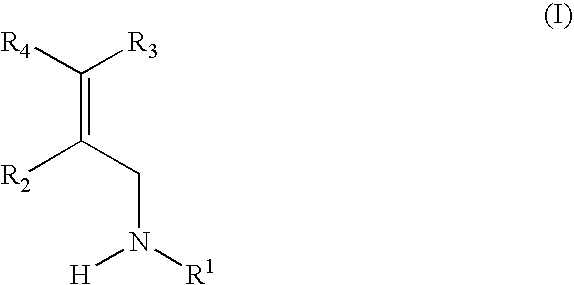
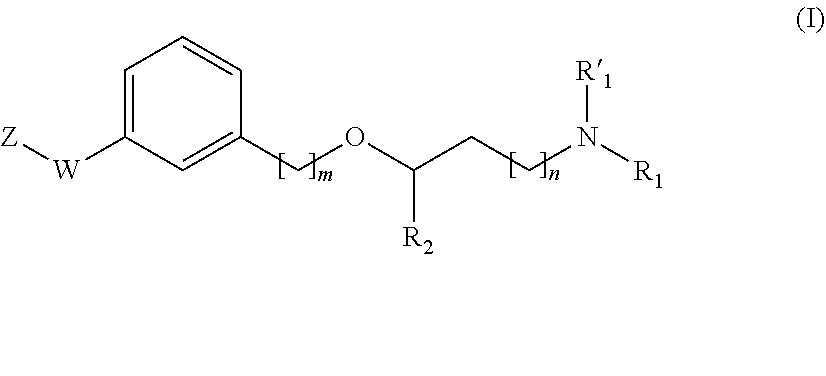
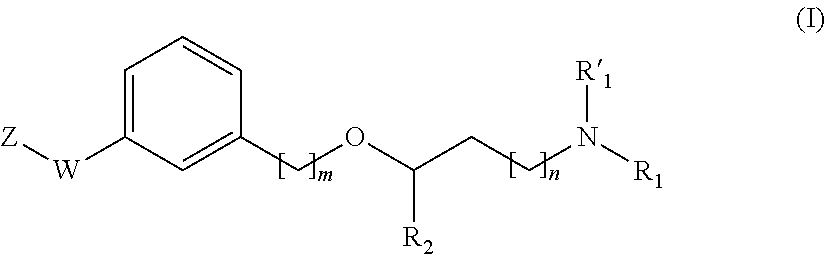
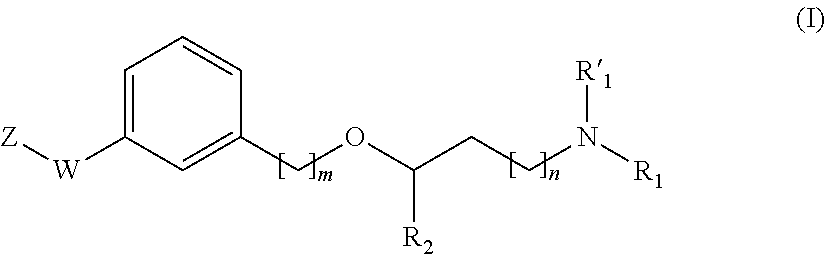
Propanamine derivatives for treating pain and pain related conditions
ActiveUS11111220B2Organic active ingredientsNervous disorderVoltage-gated calcium channel (VGCC)Adrenergic
The present invention relates to new compounds of formula (I) that show great affinity and activity towards the subunit α2δ of voltage-gated calcium channels (VGCC), especially the α2δ-1 subunit of voltage-gated calcium channels or dual activity towards the subunit α2δ of voltage-gated calcium channels (VGCC), especially the α2δ-1 subunit of voltage-gated calcium channels, and the noradrenaline transporter (NET). The invention is also related to the process for the preparation of said compounds as well as to compositions comprising them, and to their use as medicaments.
Owner:ESTEVE PHARMA SA
Naphthyloxy benzedrine derivatives and preparation method thereof
ActiveCN101955436BThe preparation method is reasonableSimple processOrganic compound preparationSexual disorderSexual impotenceSulfate radicals
The invention provides naphthyloxy benzedrine derivatives, which are prepared by subsequently reacting naphthyloxy benzedrine with acid and alkali metals or ammonium (including ammonia) compounds or amino acid or alkamine or directly reacting with acid salt; when the acid or acid salt is a sulfate radical, a naphthyloxy benzedrine sulfate complex salt is obtained; and when the acid or the acid salt is a phosphate radical, a naphthyloxy benzedrine phosphate complex salt is obtained. The method has the advantages of rational design, stable process and high production feasibility. The naphthyloxy benzedrine derivatives provided by the method have the remarkable advantages of high bioavailability, high purity, small side effect and the like. The naphthyloxy benzedrine derivatives can be prepared into a preparation, and has rapid absorption and quick response after oral administration by feeding the naphthyloxy benzedrine into blood, so that the curative effect of treating premature ejaculation and erectile dysfunction of men is better achieved. The naphthyloxy benzedrine derivatives have the structural formula shown in the description.
Owner:HANGZHOU ADAMERCK PHARMLABS INC
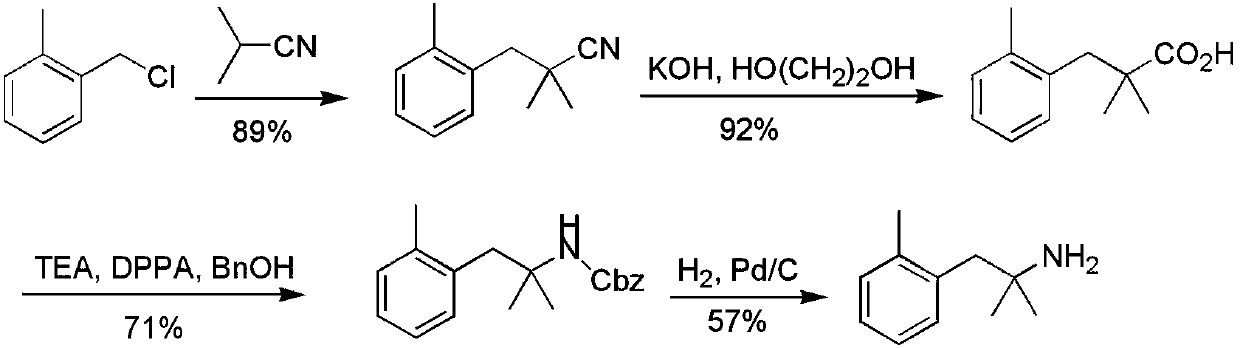
Novel synthesis method of 2-methyl-1-substituted phenyl-2-propylamine compounds
InactiveCN109678724AThe reaction route is simpleReduce usageCarboxylic acid nitrile preparationOrganic compound preparationSynthesis methodsPhenyl group
The invention discloses a novel synthesis method of 2-methyl-1-substituted phenyl-2-propylamine compounds, and belongs to the field of material synthesis. The method comprises steps as follows: substituted benzyl halide is taken as a raw material and subjected to a reaction with isobutyronitrile under the action of alkali, obtained 2-methyl-1-substituted phenyl-2-butyronitrile is hydrolyzed and subjected to reactions of Curtius rearrangement and the like, and 2-methyl-1-substituted phenyl-2-propylamine is produced. On the basis of an original method, reaction steps are shortened, the total yield is obviously increased, and meanwhile, the novel method is provided for synthesis of substituted phenyl-2-propylamine derivatives containing bromine atoms and iodine atoms.
Owner:CHANGZHOU UNIV +1
Propylamine derivative and its application in preparing tomocetin
InactiveCN100430370CThe preparation route is reasonable and feasibleSatisfactory yieldOrganic compound preparationAmino-hyroxy compound preparationBiochemical engineeringAlkoxy group
Owner:EAST CHINA UNIV OF SCI & TECH
New propanamine derivatives for treating pain and pain related conditions
InactiveUS20200207759A1Organic active ingredientsNervous disorderVoltage-gated calcium channel (VGCC)Adrenergic
The present invention relates to new compounds of general formula (I) that show dual activity towards α2δ subunit of voltage-gated calcium channels (VGCC), especially the α2δ-1 subunit, and to the noradrenallne transporter (NET). The invention is also related to the process for the preparation of said compounds as well as to compositions comprising them, and to their use as medicaments.
Owner:ESTEVE PHARMA SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com