Chemical synthesis method of (1R, 2S)-2-aryl cyclopropylamine derivative
A technology of arylcyclopropylamine and arylcyclopropylmethylamine, which is applied in the field of chemical synthesis of (1R,2S)-2-arylcyclopropylamine derivatives, and can solve the problems of low stereoselectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
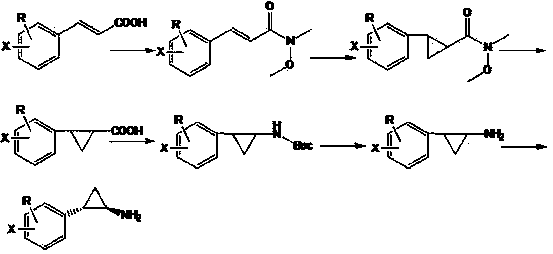
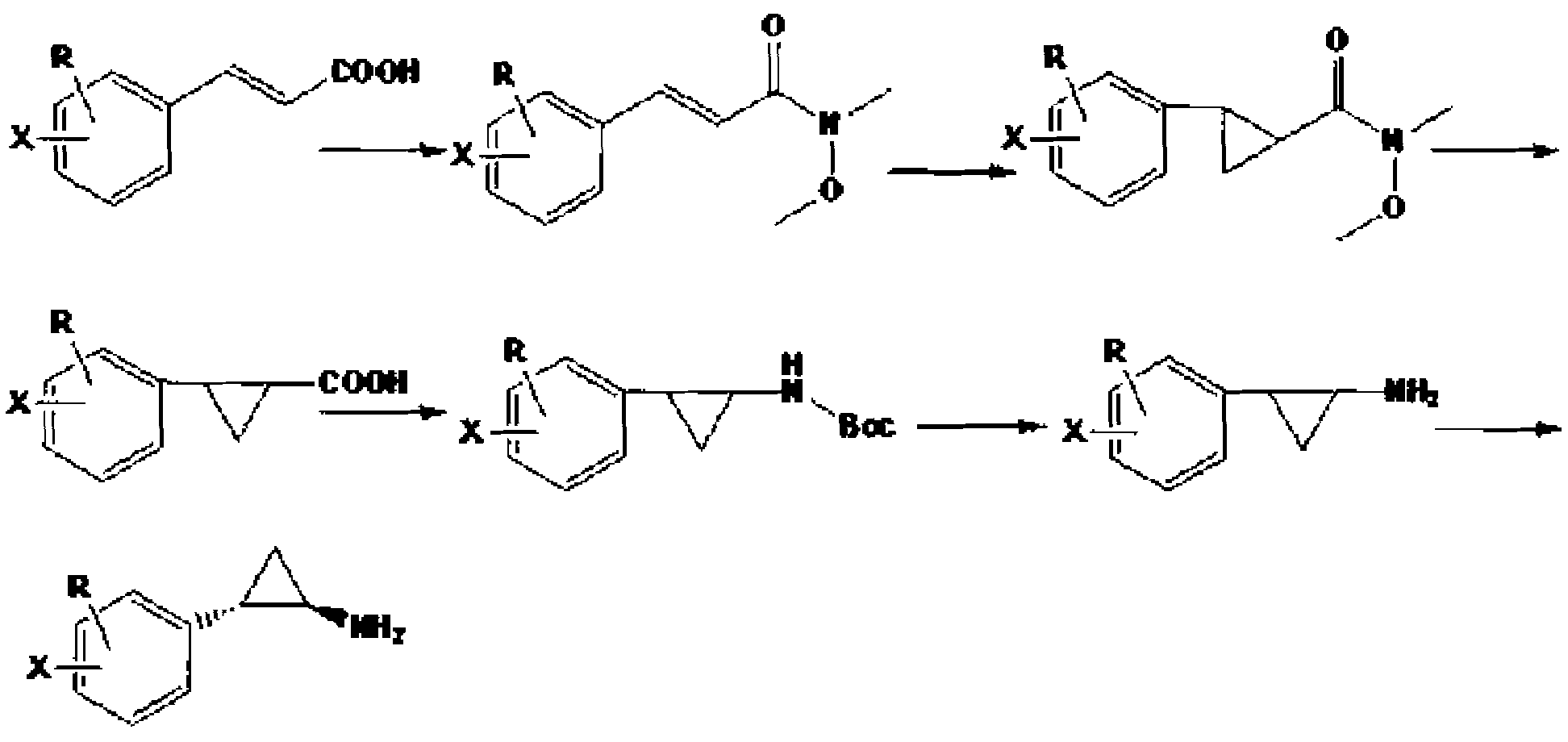
[0019] Synthesis of N-methoxy-N-methyl-3-phenylacrylamide: 50.0 g 3-phenylacrylic acid, 30.0 g triethylamine, 31.0 g N-O-dimethylhydroxylamine hydrochloride, 400 ml dichloro Add methane into a 1L reaction flask, stir mechanically for 30 min, then add 60.0 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride at room temperature overnight, wash the dichloromethane phase with water twice, and use Wash with saturated sodium bicarbonate, ammonium chloride, dilute acid and sodium chloride aqueous solution, dry and concentrate to obtain 52.0 g of light yellow N-methoxy-N-methyl-3-phenylacrylamide liquid with a yield of 80.6%.
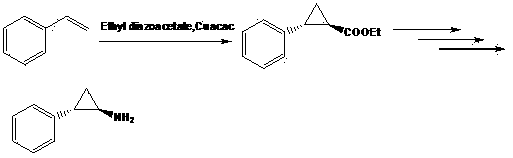
[0020] Synthesis of 2-phenyl-cyclopropylcarboxylic acid: Add 10.0 g NaH and 250 ml dimethyl sulfoxide into a three-necked flask under nitrogen protection, and add N-methoxy-N methyl-3-phenylpropene in batches amide 27.4 g, stirred and dissolved for 30 min, then added dropwise 42.0 g trimethylsulfoxide iodide, and reacted at room temperature. After...
Embodiment 2
[0024] Synthesis of N-methoxy-N-methyl-3-(4'-methoxyphenyl)acrylamide: 60.0 g 3-(4'-methoxyphenyl)acrylic acid, 33.5 g triethylamine , 32.7 g of N-O-dimethylhydroxylamine hydrochloride, 400 ml of dichloromethane were added to a 1L reaction flask, mechanically stirred for 30 min, and then 62.0 g of 1-ethyl-3-(3-dimethylaminopropyl) carbodisulfide was added Amine hydrochloride at room temperature overnight, washed with dichloromethane phase twice, washed with saturated sodium bicarbonate, ammonium chloride, dilute acid, sodium chloride aqueous solution, dried and concentrated to obtain yellow N-methoxy-N methyl-3 -(4'-methoxyphenyl)acrylamide liquid 55.3 g, yield 74.3%.
[0025] Synthesis of 2-(4'-methoxyphenyl)-cyclopropanecarboxylic acid: Add 10.0 g NaH and 300 ml dimethyl sulfoxide into a three-necked flask under nitrogen protection, and add N-methoxy-N 31.7 g of methyl-3-(4'-methoxyphenyl)acrylamide was stirred and dissolved for 30 min, then 42.0 g of trimethylsulfoxide iod...
Embodiment 3
[0029] Synthesis of N-methoxy-N-methyl-3-(4'-bromophenyl)acrylamide: 75.0 g 3-(4'-bromophenyl)acrylic acid, 33.0 g triethylamine, 32.7 g N-O -Add dimethylhydroxylamine hydrochloride and 400 ml of dichloromethane into a 1L reaction flask, stir mechanically for 30 min, then add 64.0 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride Overnight at room temperature, the dichloromethane phase was washed twice with water, washed with saturated sodium bicarbonate, ammonium chloride, dilute acid, sodium chloride aqueous solution, dried and concentrated to obtain yellow N-methoxy-N methyl-3-(4' -Bromophenyl) acrylamide liquid 75.4 g, the yield is 84.5%.
[0030]Synthesis of 2-(4'-bromophenyl)-cyclopropanecarboxylic acid: Add 10.0 g NaH and 350 ml dimethyl sulfoxide into a three-necked flask under nitrogen protection, and add N-methoxy-N methyl - 38.7 g of 3-(4'-bromophenyl) acrylamide, stirred and dissolved for 30 min, then added dropwise 42.0 g of trimethylsulfoxide iodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com