A kind of efficient preparation method of heterocyclic drug intermediate
A technology of heterocycles and intermediates, applied in the field of biocatalysis, can solve the problems of increasing the production cost of catalysts, adding more enzymes and organic solvents, unfavorable amplification and application, etc., and achieves the effect of reducing the product inhibition effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Rational mutation and mutant construction of key amino acids of alcohol dehydrogenase KpADH include the following steps:
[0044] 1. Identify key amino acids that control stereoselectivity and catalytic activity
[0045] Through the crystal structure (PDB: 5Z2X) derived from Kluyveromyces alcohol dehydrogenase KpADH, find out the amino acid that interacts with the substrate NBPO; through the conservative analysis of amino acid sequence alignment with more than 30% homology, screen out Key amino acid residue 127.
[0046] 2. Construction of Mutants
[0047] Using the pET28a-KpADH recombinant plasmid preserved in the laboratory as a template (recorded in the patent application with publication number CN105936909A), the 127th amino acid of the alcohol dehydrogenase KpADH with amino acid sequence such as SEQ ID No.1 was carried out by using the whole plasmid PCR method Site-directed saturation mutagenesis. The mutants Y127A, Y127C, Y127F, Y127I, Y127M, Y127Q, Y127V, Y127...
Embodiment 2
[0049] According to Example 1, the mutants with improved catalytic activity were induced, expressed, purified and kinetically determined, including the following steps:
[0050] 1. Induced expression
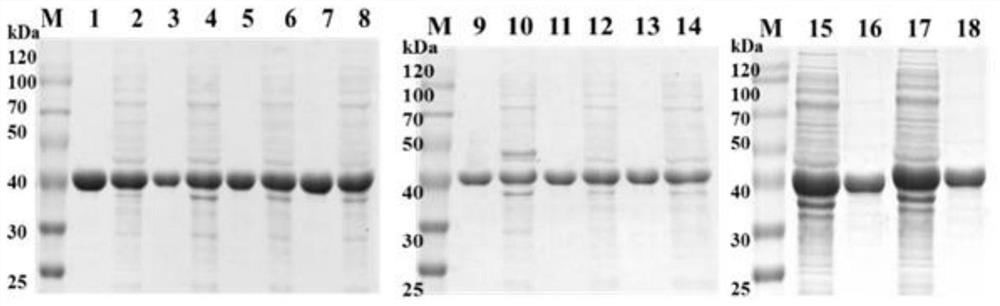
[0051] The mutant in Example 1 was inoculated into 50 μL / mL LB medium, and cultured with shaking at 37° C. and 180 rpm. When OD600 reached 0.8, isopropyl-β-D-thiogalactopyranoside (IPTG, 0.2M) was added to a final concentration of 0.2mM, and the temperature was lowered to 25°C to induce protein expression. At the end of the culture, cells were harvested by centrifugation and sonicated in PBS buffer (pH 7.4). The cell lysate was centrifuged at 8000rpm for 30min.
[0052] 2. Protein purification
[0053] Nickel column affinity chromatography is used. According to the His-Tag tag at the N-terminal of KpADH, it can competitively bind with nickel, and gradient or linear elution methods can be used. KpADH and its mutants could be completely eluted when the imidazole concentration ...
Embodiment 3
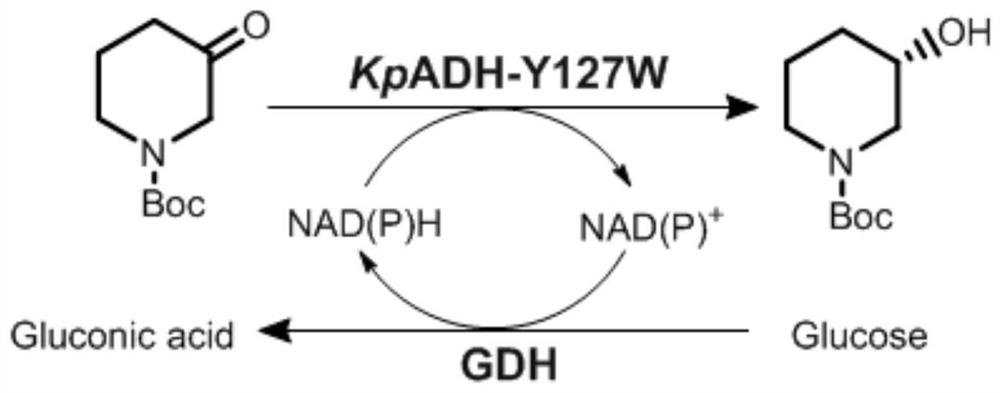
[0061] In order to explore the heterocyclic ketone substrate spectrum of KpADH and its mutants, the heterocyclic ketones dihydro-3(2H)-furanone, tetrahydrothiophen-3-one, cyclohexanone, 4-ethylcyclohexanone, N -Boc-3-pyrrolidone, N-Boc-2-piperidone, N-Boc-3-piperidone, N-Boc-4-piperidone were used to determine the substrate kinetics of KpADH and mutants to heterocyclic ketones study. As shown in Table 2, except for the N-Boc-2-piperidone substrate in the ortho position, it exhibits catalytic activity for other substrates. Mutant Y127W in the substrate spectrum, the k of the substrate N-Boc-3-pyrrolidone cat / K m from 0.2s -1 mM -1 increased to 2.1s -1 mM -1 , and the k for substrate 7a cat / K m from 3.6s -1 mM -1 increased to 31.0s -1 mM -1. At the same time, the e.e. values for the two substrates were also increased to over 99%. This indicated that the mutant Y127W specifically increased the catalytic efficiency of substrates with a Boc group and a carbonyl gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com