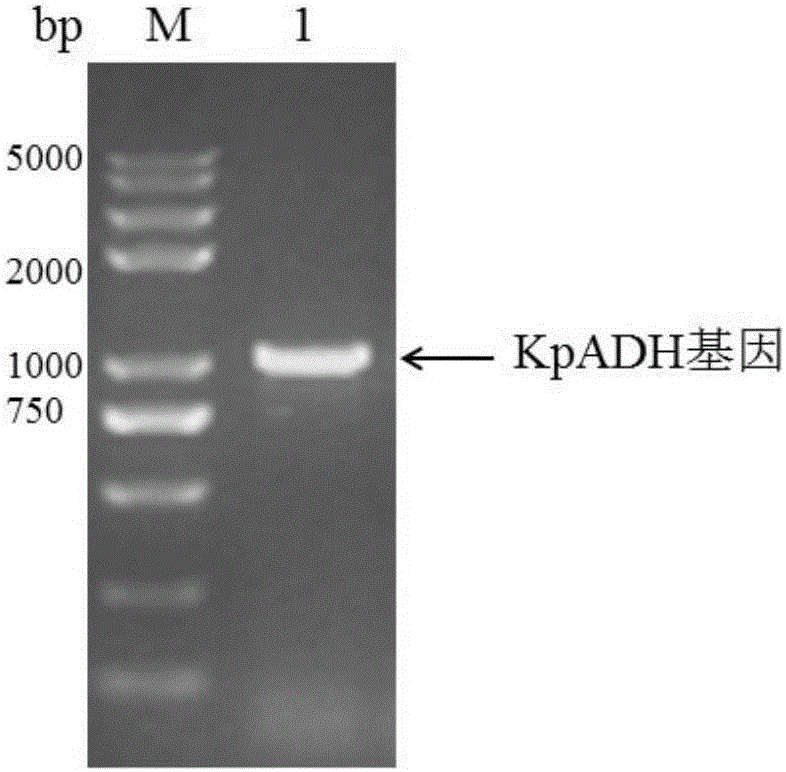
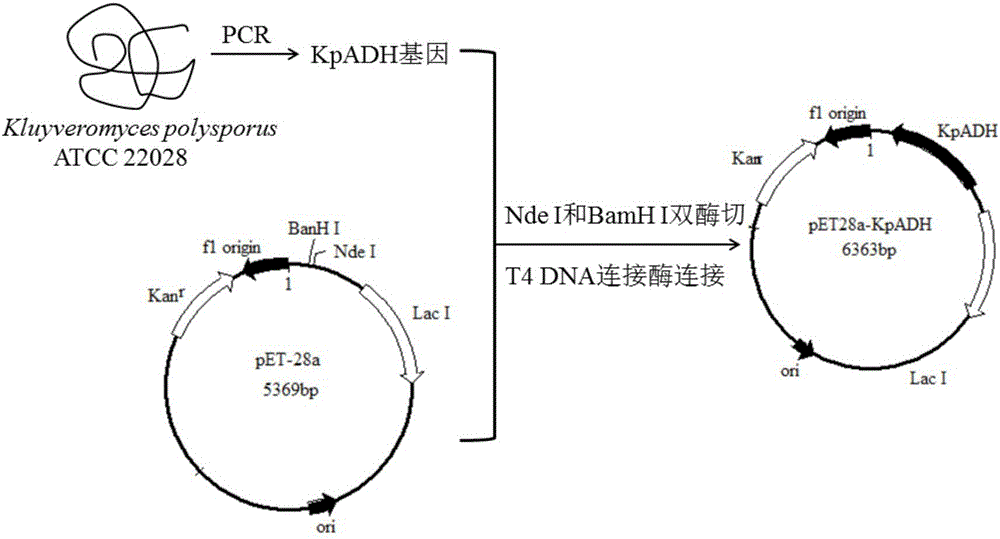
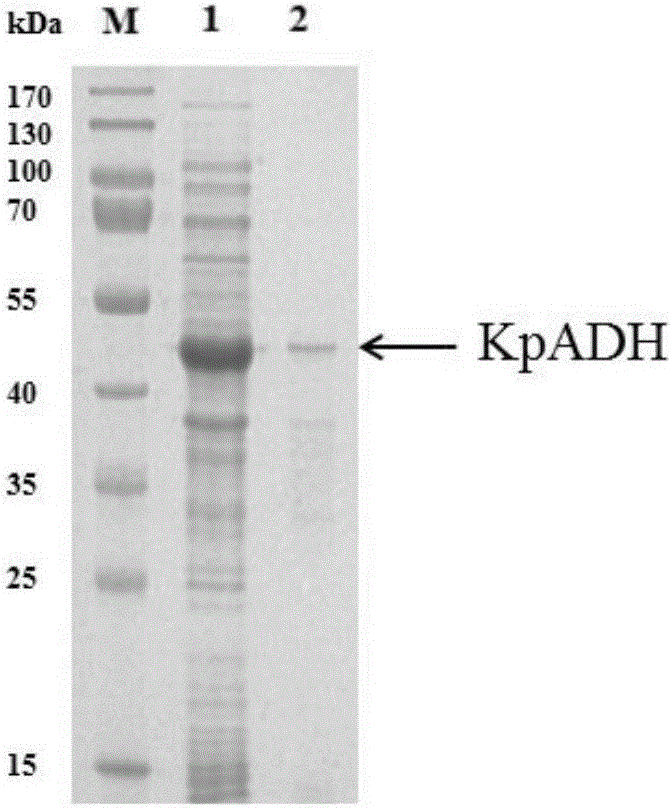
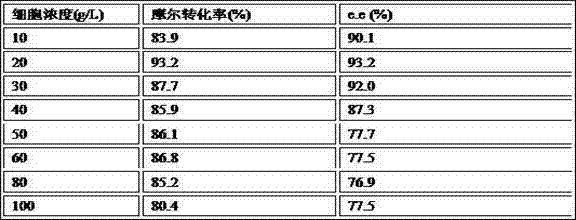
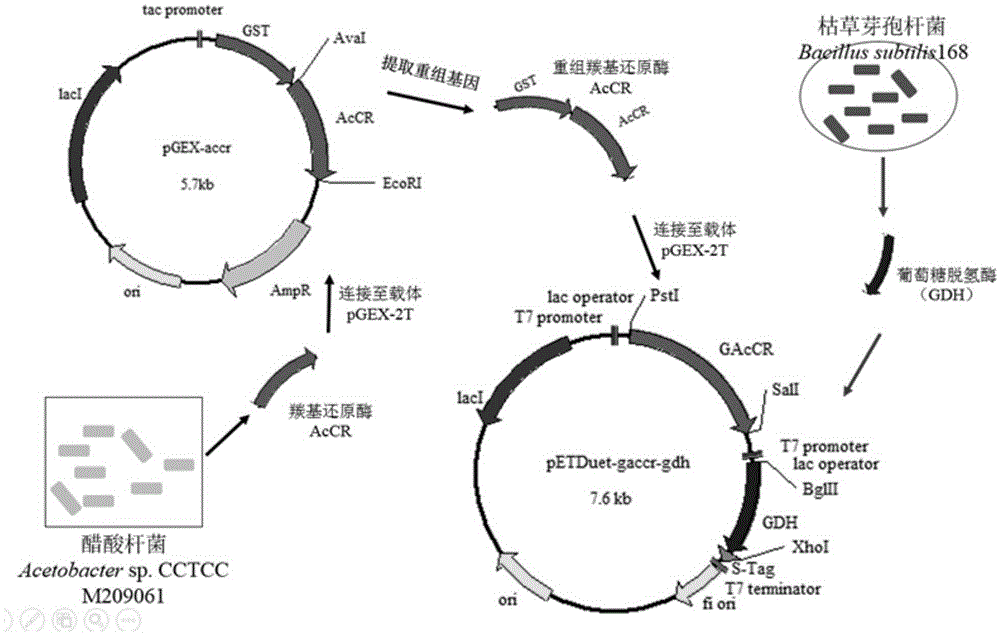
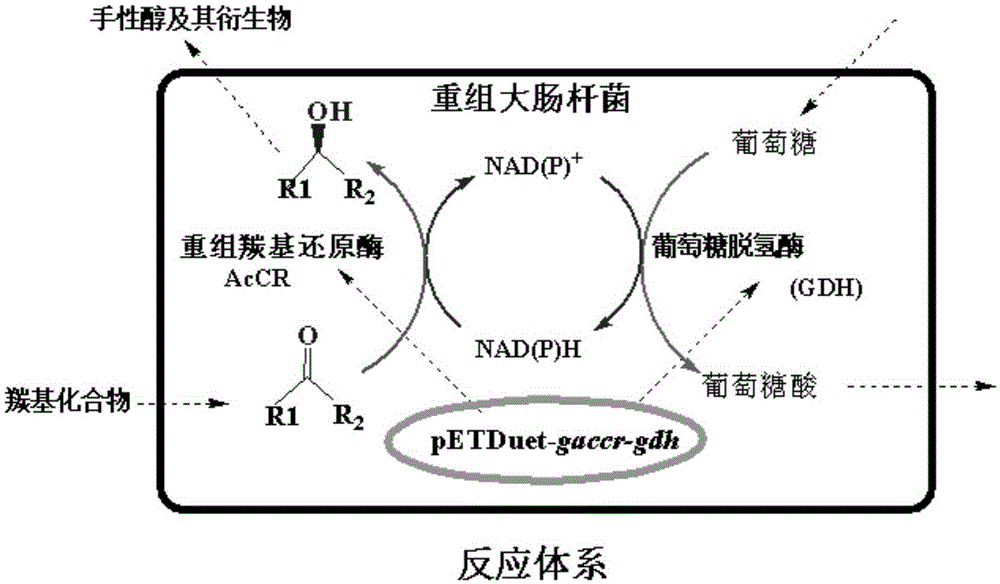
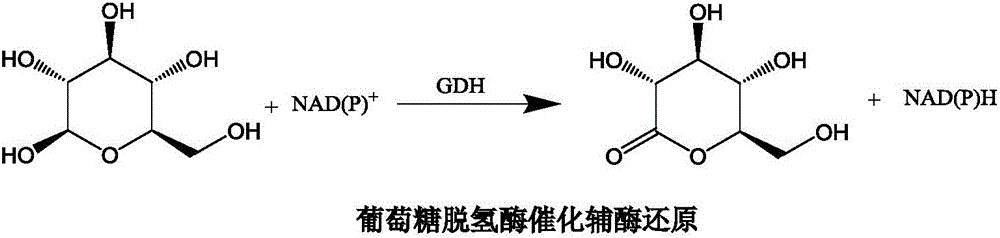
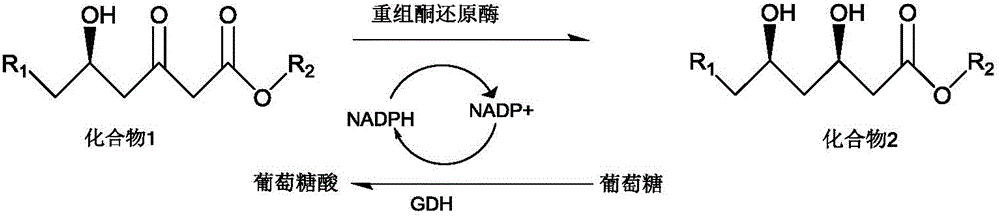
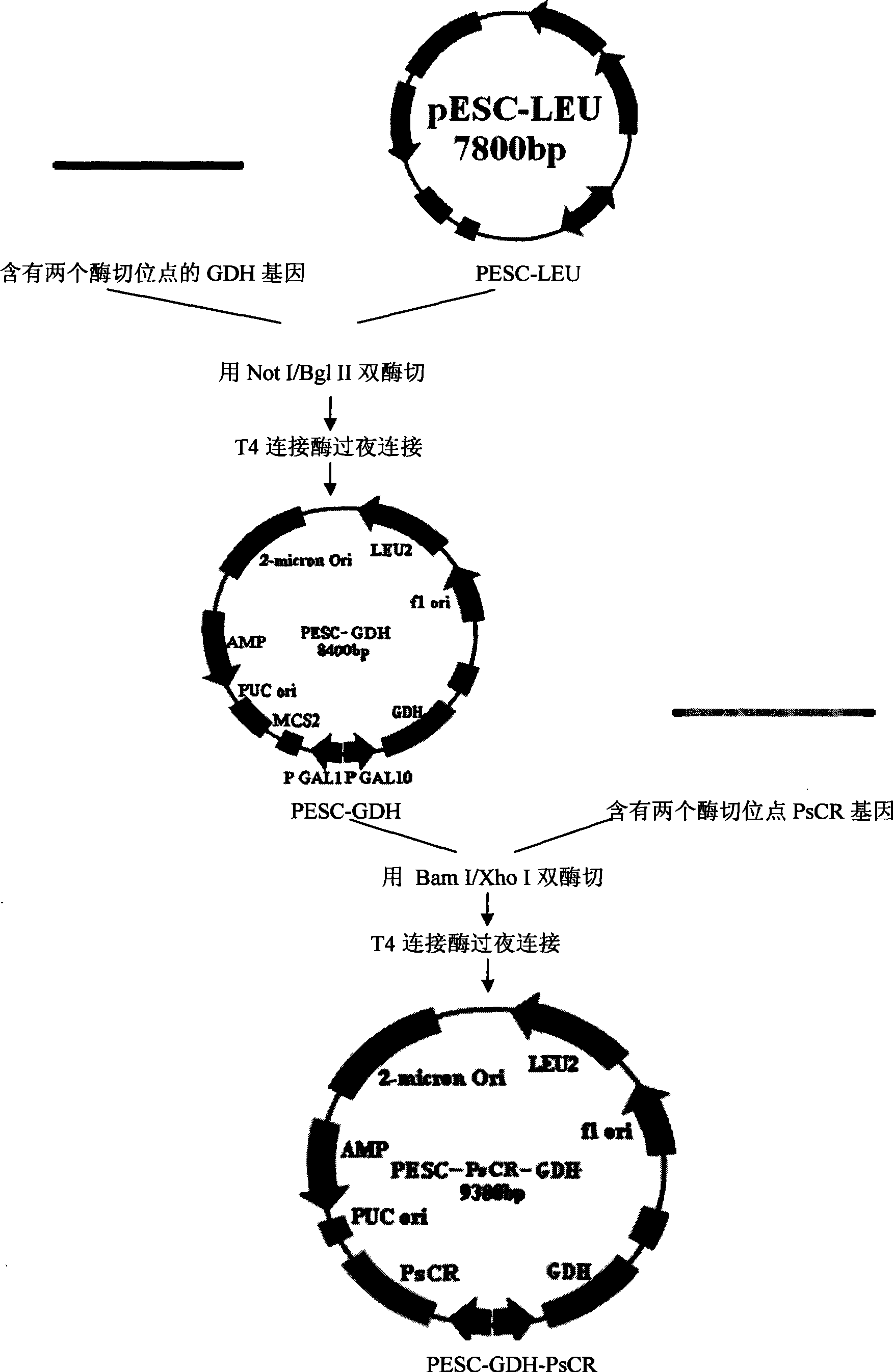
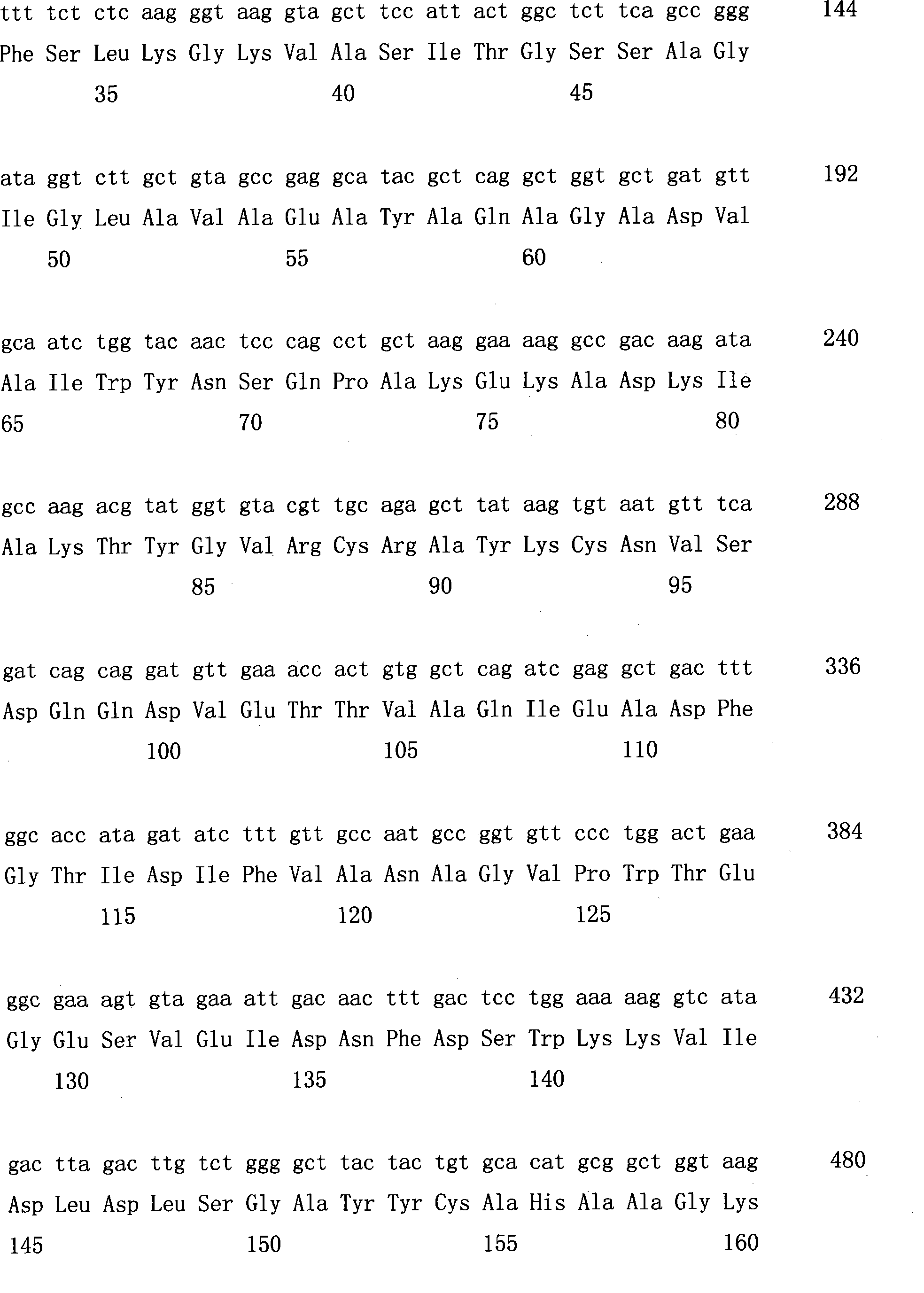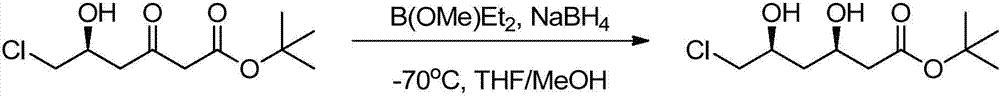Patents
Literature
251 results about "Glucose dehydrogenase (pyrroloquinoline-quinone)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biosensor
InactiveUS20050175509A1Eliminate measurement errorsHigh precisionMicrobiological testing/measurementBiological material analysisCompound (substance)Organic compound
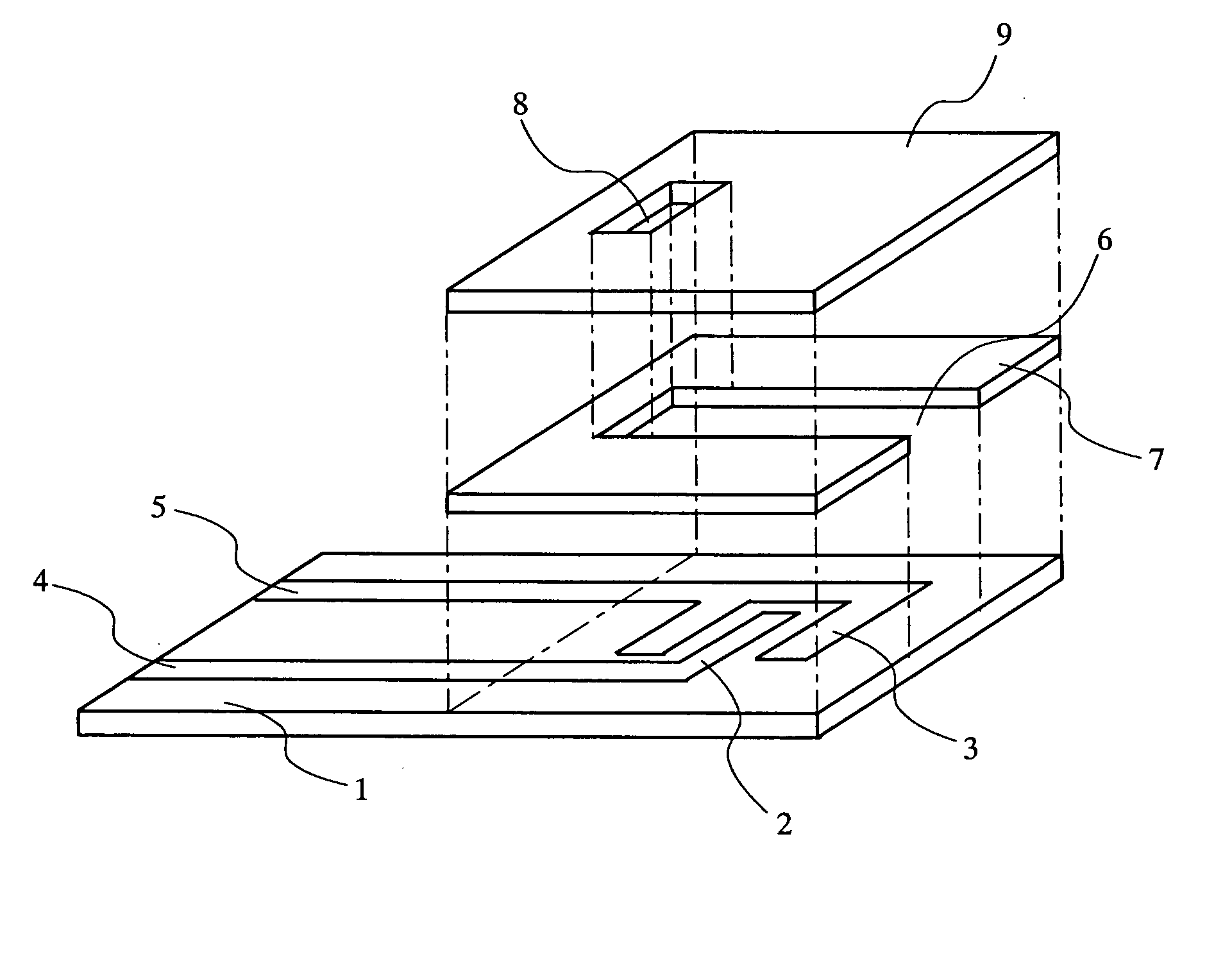
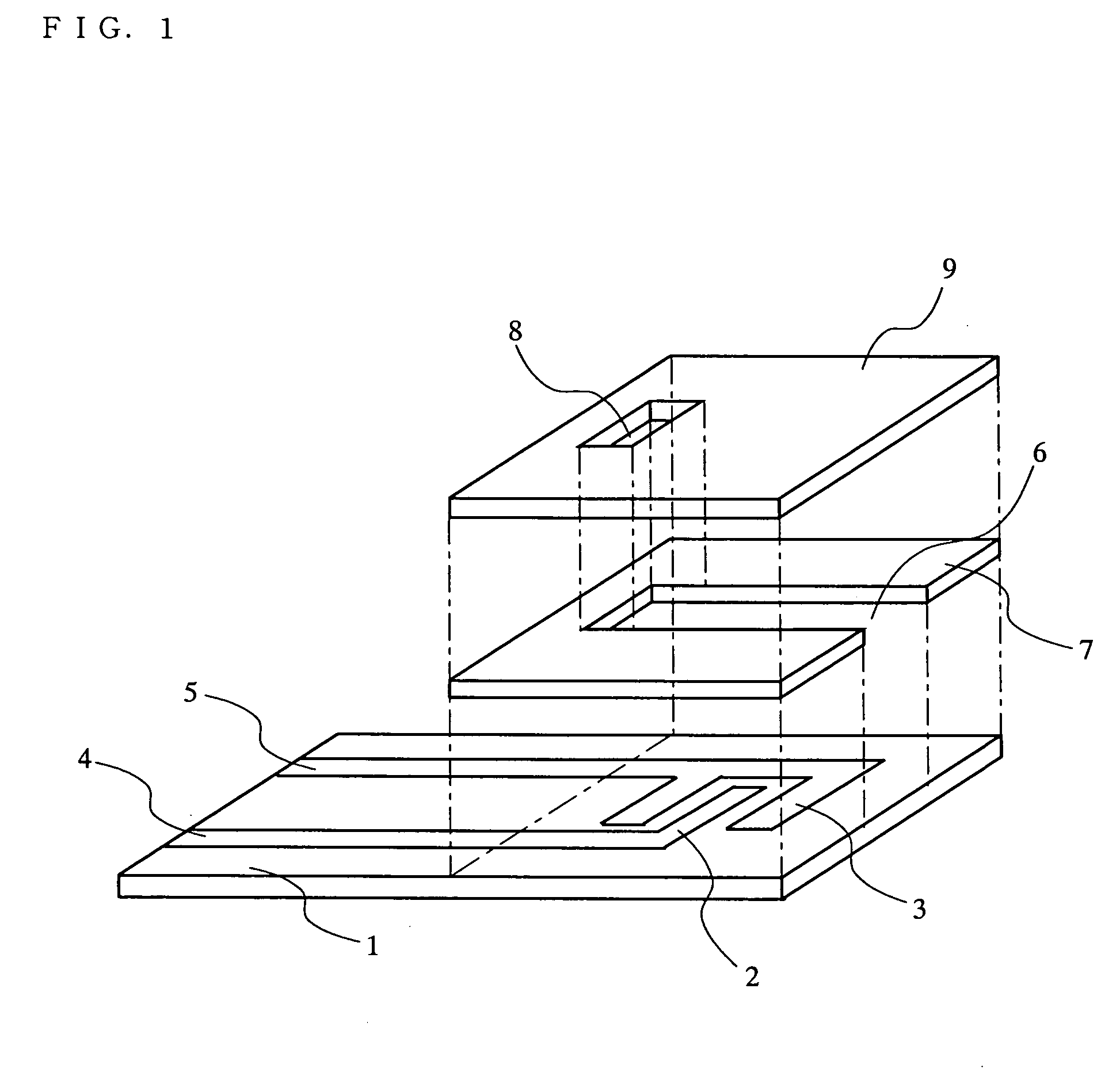
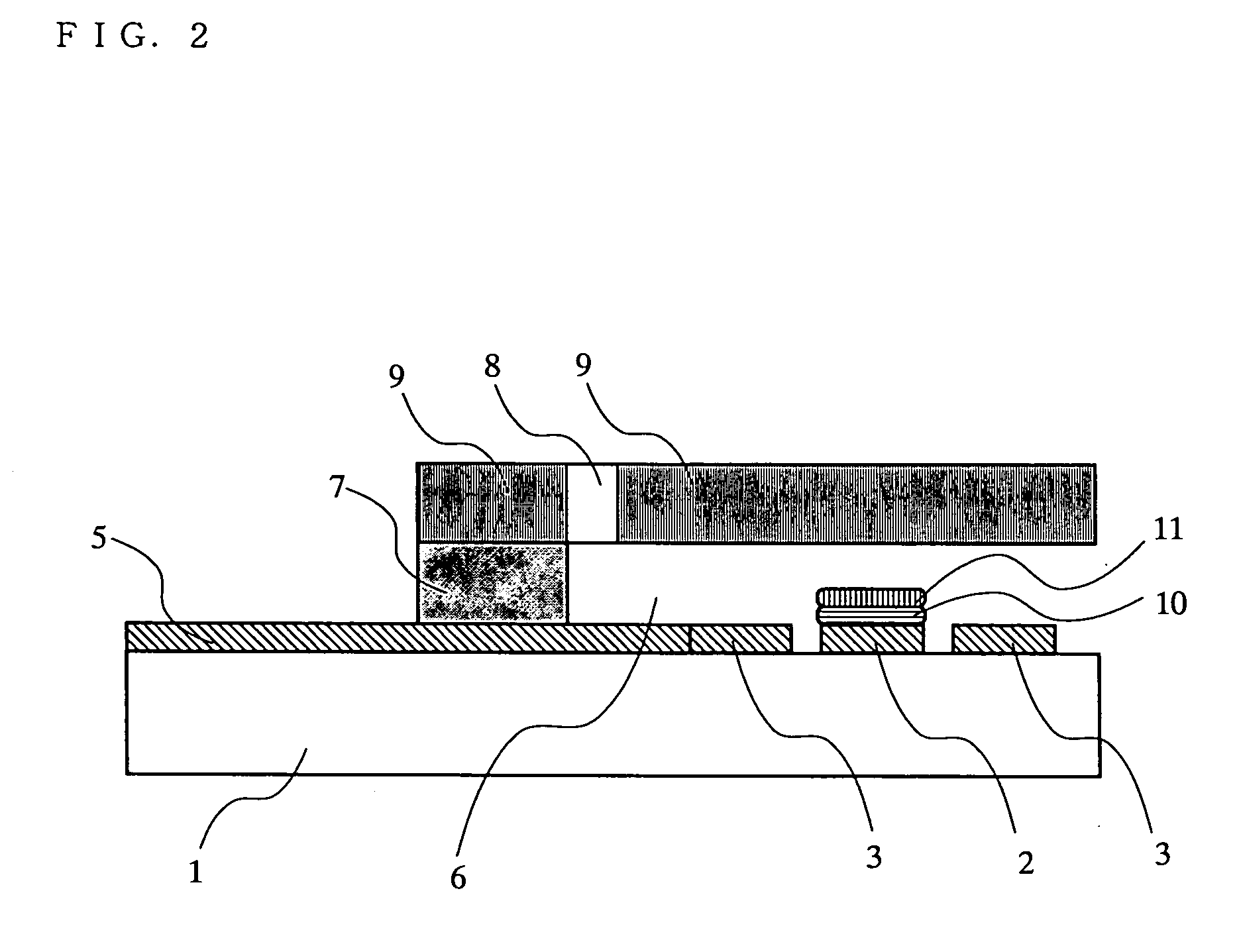
The present invention relates to a biosensor which comprises an electrode system including at least one pair of electrodes, at least one insulating base plate for supporting the electrode system, a first reaction layer provided at least on a working electrode of the electrode system, including an organic compound having a functional group capable of bonding or being adsorbed to an electrode and a hydrophobic hydrocarbon group, a second reaction layer provided on the first reaction layer, including an amphiphilic lipid capable of bonding or being adsorbed to a hydrophobic portion of the first reaction layer, and a reagent system carried in a two-component membrane composed of the first and second reaction layers, including at least membrane-binding type pyrroquinoline quinone-dependent glucose dehydrogenase and an electron mediator.
Owner:PANASONIC CORP
E. coli transformant, method for producing flavin-bound glucose dehydrogenase using the same, and mutant flavin-bound glucose dehydrogenases
ActiveUS20130309750A1Accurate measurementEfficiently obtainedBacteriaOxidoreductasesEscherichia coliMicroorganism
A flavin-bound glucose dehydrogenase (FAD-GDH) with high substrate specificity for D-glucose. A gene encoding a mutant FAD-GDH with its N-terminal region, containing an amino acid sequence corresponding to MKITAAIITVATAFASFASA that exists in the N-terminal region, deleted from the amino acid sequence of a wild-type FAD-GDH derived from Mucor is introduced into E. coli to obtain an E. coli transformant. Subsequently, this E. coli transformant is cultured to obtain an FAD-GDH with a specific N-terminal region deleted. The transformant allows the production of a large amount of GDH in a short time as compared with the original microorganism. An FAD-GDH that is less susceptible to the effects of dissolved oxygen and allows accurate measurement of glucose even in the presence of sugar compounds other than glucose in a sample.
Owner:KIKKOMAN CORP
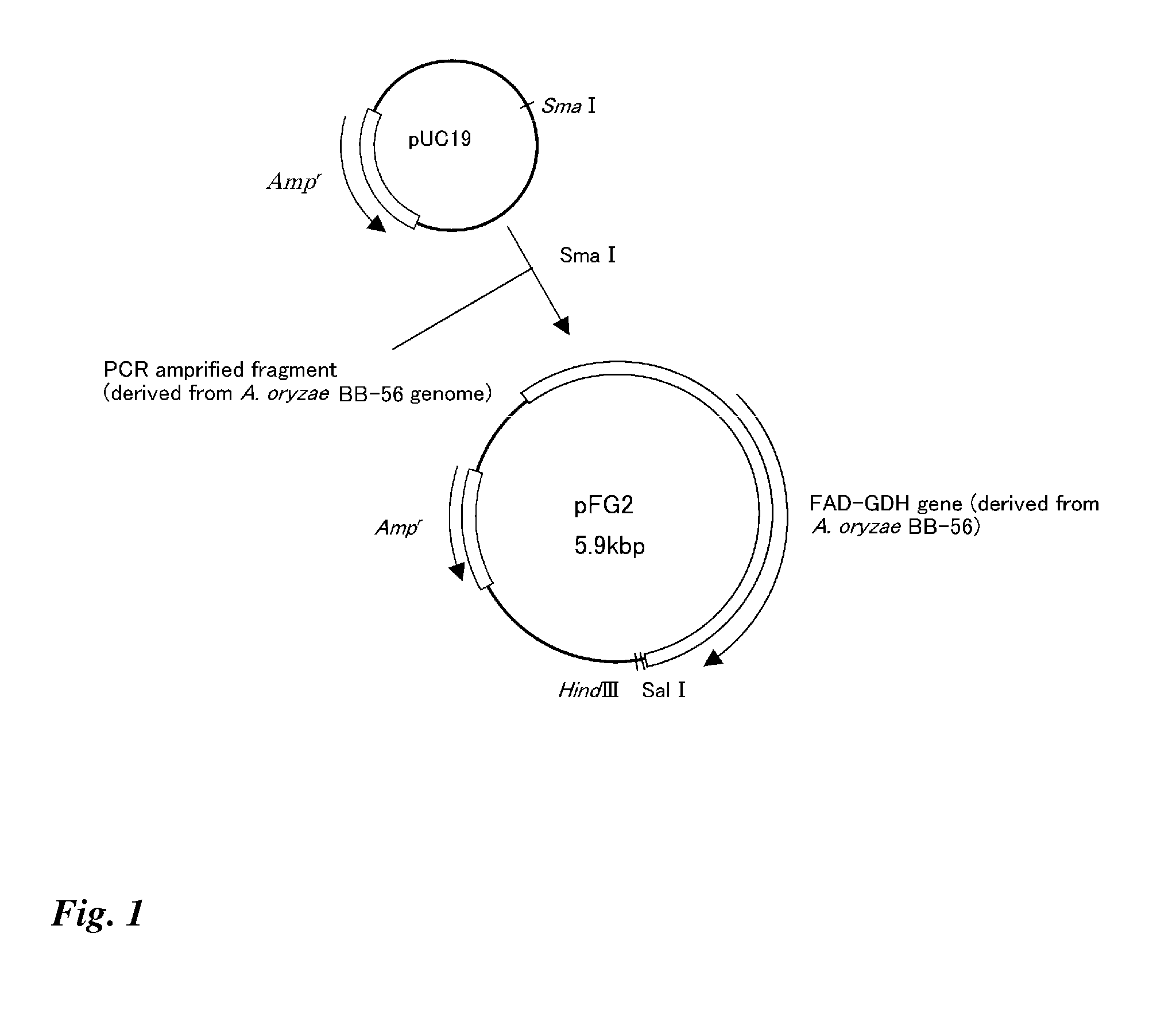
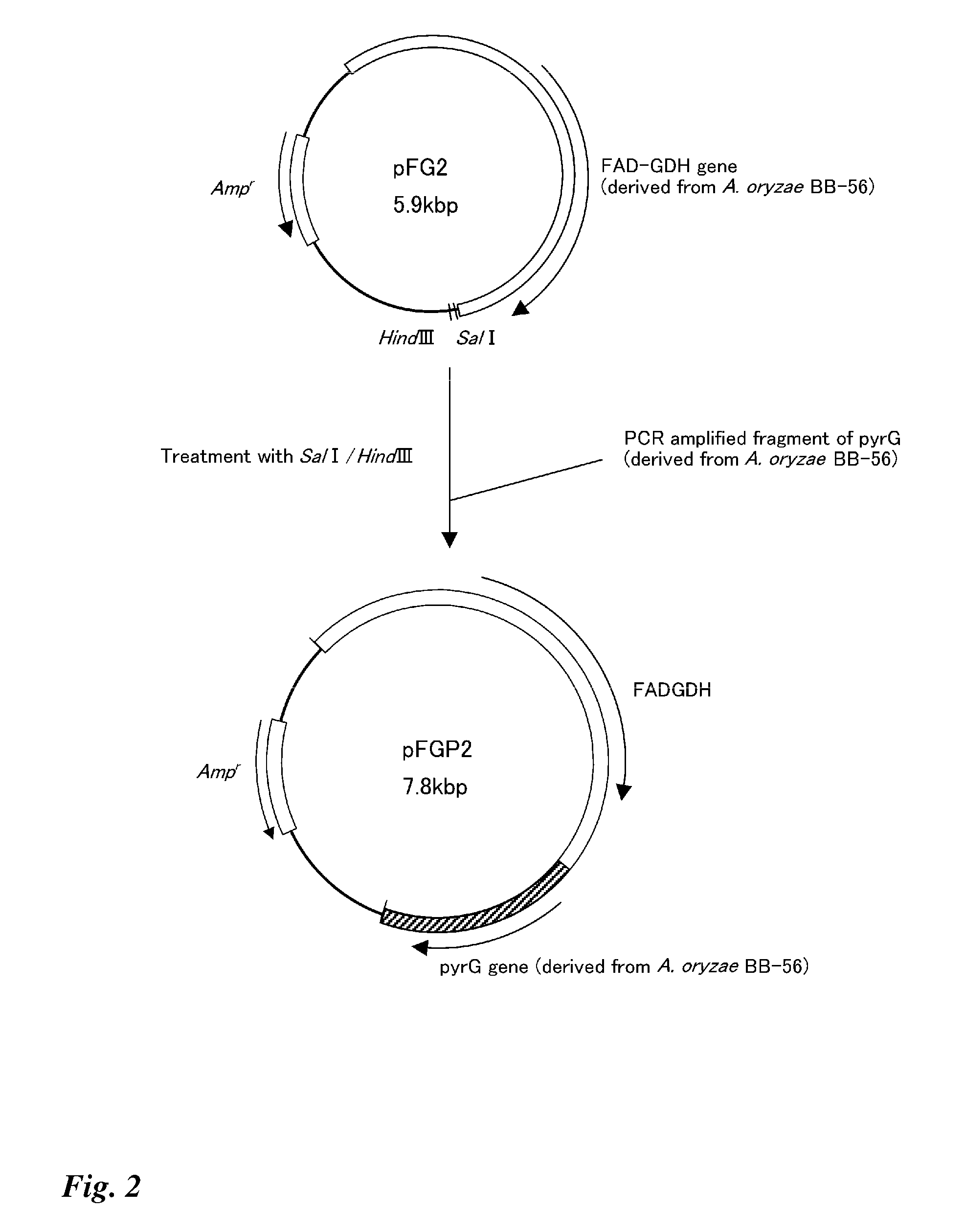
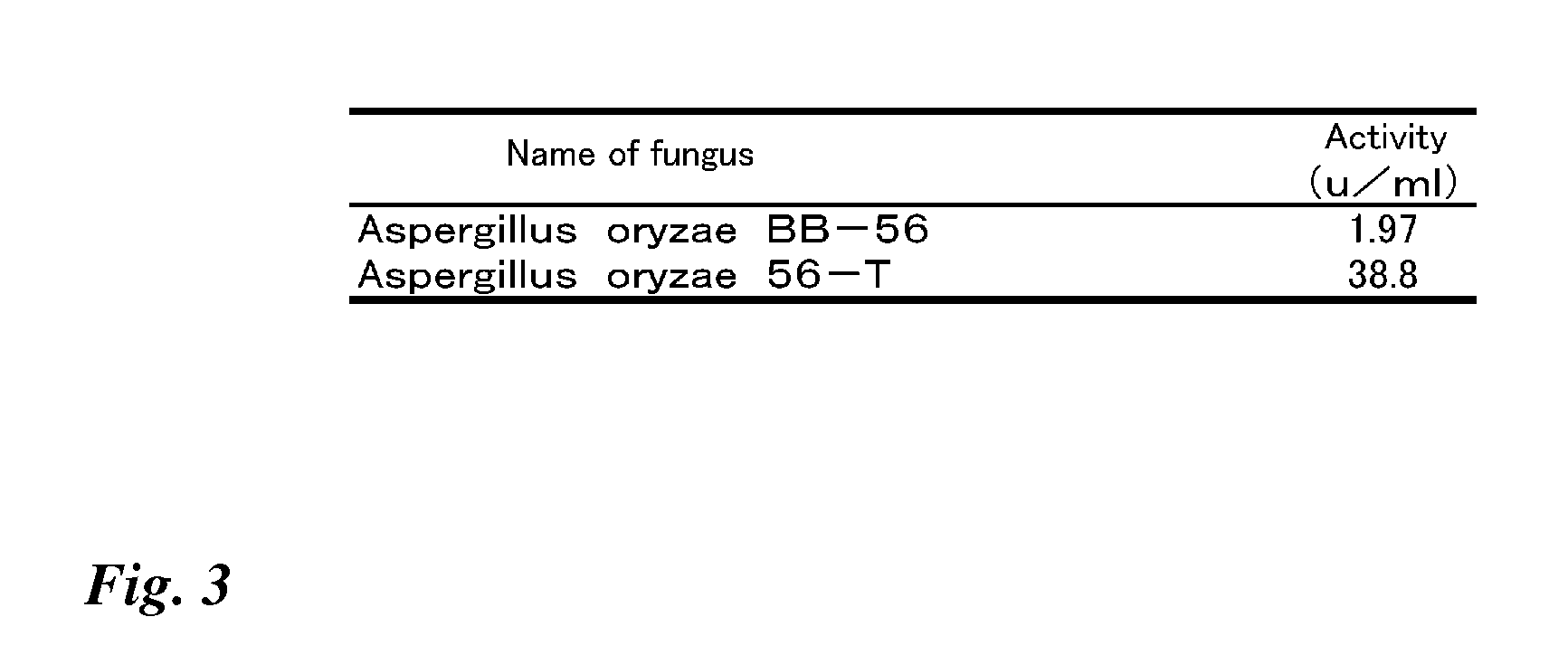
Method for producing glucose dehydrogenase from aspergillus oryzae
ActiveUS20080014612A1Efficient productionStable productionSugar derivativesBacteriaAspergillus oryzaeMicrobiology
The present invention effectively produces glucose dehydrogenase derived from Aspergillus oryzae, and provides more practical glucose dehydrogenase. The invention makes it possible to efficiently produce glucose dehydrogenase and to obtain glucose dehydrogenase in more practical manner by using a glucose dehydrogenase gene isolated from Aspergillus oryzae.
Owner:TOYOBO CO LTD
FAD-conjugated glucose dehydrogenase gene
InactiveUS8492130B2Maintain good propertiesEasy to identifyBacteriaSugar derivativesNucleotidePolynucleotide
An object of the present invention is to provide: a novel gene (polynucleotide) encoding an FAD-conjugated glucose dehydrogenase having excellent properties that it has excellent reactivity to glucose, excellent thermal stability, and excellent substrate-recognition performance and also has a low activity for maltose; a process for the production of the enzyme using a transformant cell transfected with the gene; and a method for the determination of glucose, a reagent composition for use in the determination of glucose, a biosensor for use in the determination of glucose and others, each characterized by using the enzyme obtained. The invention relates to a polynucleotide encoding an FAD-conjugated glucose dehydrogenase, comprising a polypeptide containing an amino acid sequence: X1-X2-X3-X4-X5-X6 (wherein X1 and X2 independently represent an aliphatic amino acid residue; X3 and X6 independently represent a branched amino acid residue; and X4 and X5 independently represent a heterocyclic amino acid residue or an aromatic amino acid residue); and others.
Owner:PHC CORP
Method for producing glucose dehydrogenase from Aspergillus oryzae
Owner:TOYOBO CO LTD
Glucose dehydrogenase and process for producing the dehydrogenase
InactiveUS7741090B2Increased substrate specificityImprove thermal stabilityBacteriaPeptide/protein ingredientsBiotechnologyMicroorganism
A novel glucose dehydrogenase, which is an enzyme that has high substrate specificity, can be produced at a low cost, is not affected by oxygen dissolved in a measurement sample and, in particular, has superior thermal stability is obtained by culturing a microorganism belonging to the genus Burkhorderia and having glucose dehydrogenase producing ability in a medium and collecting glucose dehydrogenase from the medium and / or cells of the microorganism.
Owner:SODE
Fad-conjugated glucose dehydrogenase gene
InactiveUS20110033880A1Maintain good propertiesReduced activityFungiSugar derivativesNucleotidePolynucleotide
An object of the present invention is to provide: a novel gene (polynucleotide) encoding an FAD-conjugated glucose dehydrogenase having excellent properties that it has excellent reactivity to glucose, excellent thermal stability, and excellent substrate-recognition performance and also has a low activity for maltose; a process for the production of the enzyme using a transformant cell transfected with the gene; and a method for the determination of glucose, a reagent composition for use in the determination of glucose, a biosensor for use in the determination of glucose and others, each characterized by using the enzyme obtained. The invention relates to a polynucleotide encoding an FAD-conjugated glucose dehydrogenase, comprising a polypeptide containing an amino acid sequence: X1-X2-X3-X4-X5-X6 (wherein X1 and X2 independently represent an aliphatic amino acid residue; X3 and X6 independently represent a branched amino acid residue; and X4 and X5 independently represent a heterocyclic amino acid residue or an aromatic amino acid residue); and others.
Owner:PHC CORP
Alcohol dehydrogenase, gene and recombinase thereof, and application of alcohol dehydrogenase in synthesis of chiral diaryl secondary alcohol
InactiveCN105936909AImprove expression levelIncrease enzyme activityOxidoreductasesGenetic engineeringDiaryl ketoneGenus Kluyveromyces
The invention discloses an alcohol dehydrogenase, a gene thereof, a recombinant expression vector and a recombinant expression transformant respectively containing the gene, a recombinase of the alcohol dehydrogenase, and an application of the alcohol dehydrogenase in asymmetric reduction synthesis of chiral diaryl secondary alcohol as a catalyst, and belongs to the technical field of bioengineering. The alcohol dehydrogenase is from Kluyveromyces sp. CCTCCM2011385, has a carbonyl group reduction function, and also has a hydroxy group oxidation function. Extra addition of glucose dehydrogenase and other enzymes used for cofactor circulation is not needed when the alcohol dehydrogenase is used in the reduction of diaryl ketone into the chiral diaryl secondary alcohol as a biocatalyst, and the alcohol dehydrogenase has the advantages of high catalysis efficiency, mild reaction conditions, easy product recovery and low cost, so the alcohol dehydrogenase has very good application and exploitation prospect in the production of antihistamine medicines.
Owner:JIANGNAN UNIV
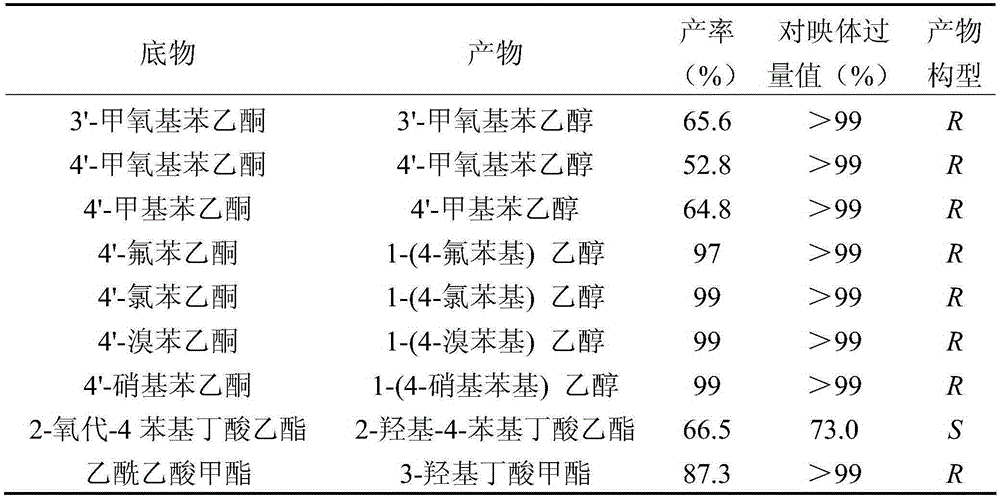
Method for preparing (R)-2-hydroxy-4-phenyl ethyl butyrate by catalyzing with recombinant carbonyl reductase
InactiveCN102618590AAvoid inhibitionImprove conversion abilityMicroorganism based processesFermentationEscherichia coliEthyl butyrate
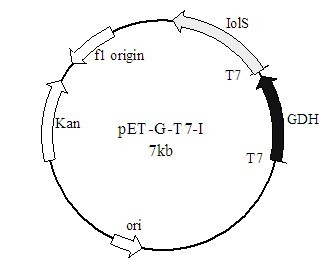
The invention discloses a method for preparing (R)-2-hydroxy-4-phenyl ethyl butyrate by catalyzing recombinant carbonyl reductase, which belongs to the technical field of biological engineering. The method comprises the following steps of: cloning gene segments of carbonyl reductase (IolS) and glucose dehydrogenase (GDH) from bacillus subtilis CGMCC NO.1.1508, expressing an IolS gene and a GDH gene in series by adopting a dual-starter method to construct a recombinant plasmid pET24a-G-T7-I, and introducing the plasmid into escherichia coli BL21(DE3); and under the condition of not adding or adding a small amount of NADP+cofactors, performing biotransformation by taking a cell-free extract of the escherichia coli recombinant plasmid as a catalyst, 2-oxo-4-phenyl ethyl butyrate as a substrate and glucose as a substrate to obtain (R)-2-hydroxy-4-phenyl ethyl butyrate, wherein the enantiomeric excess value of the product is higher than 99.5 percent. In the method, IolS and GDH are co-expressed, so that efficient regeneration of an intra-cellular cofactor NADP(H) is realized, production cost is lowered, and a good industrial application prospect is achieved.
Owner:JIANGNAN UNIV
Flavin-binding glucose dehydrogenases
ActiveUS20110318810A1Accurate measurementAccurate blood glucose levelSugar derivativesBacteriaMicroorganismLactose
A flavin-binding glucose dehydrogenase with a high substrate specificity for D-glucose. The flavin-binding glucose dehydrogenase which is derived from a microorganism belonging to the genus Mucor. The flavin-binding glucose dehydrogenase has a low reactivity for maltose, D-galactose and D-xylose compared to its reactivity for D-glucose, and therefore is relatively unaffected by these saccharide compounds. The flavin-binding glucose dehydrogenase is also relatively unaffected by dissolved oxygen, and allows accurate measurement of glucose amounts even in the presence of saccharide compounds other than glucose in samples.
Owner:KIKKOMAN CORP
Glucose Dehydrogenase/Cytochrome Fusion Protein
A fusion protein of pyrroloquinoline quinone glucose dehydrogenase (PQQGDH) and a cytochrome is disclosed. PQQGDH is, for example, a water-soluble PQQGDH derived from Acinetobacter calcoaceticus. The cytochrome is, for example, an electron transfer domain of quinohemoprotein ethanol dehydrogenase from Comamonas testosteroni. The fusion protein of the present invention shows intramolecular electron transfer from PQQ, a redox center, to the cytochrome, which allow construction of a direct electron transfer-type glucose sensor which requires no electron mediators.
Owner:ARKRAY INC
Method for biologically preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester with recombinant escherichia coli expressed ketoreductase
InactiveCN103173503AHigh optical purityGenerate efficientlyMicroorganism based processesFermentationHydroxybutyric acidEthyl acetate
The invention discloses a method for biologically preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester with recombinant escherichia coli expressed ketoreductase and application thereof in preparing (S)-4-chloro-3-hydroxy butyric acid ethyl ester through asymmetric reduction of 4-chloroacetoacetic acid ethyl ester. The recombinant escherichia coli for expressing the gene of the ketoreductase (KRED) is coupled with glucose dehydrogenase (GDH) so that the escherichia coli cell is recombined into a catalyst; efficient preparation of (S)-4-chloro-3-hydroxy butyric acid ethyl ester through asymmetric reduction of 4-chloroacetoacetic acid ethyl ester in a single water phase is realized no matter in the presence of or in the absence of coenzyme NAD(P)H; and the molar transformation rate is higher than 92% and the enantiomer excess (e.e.) of the product is 100%.
Owner:JIANGXI NORMAL UNIV +1
Carbonyl reductase AcCR and encoding gene and application thereof
InactiveCN105349503ACan be catalyzed to generateAchieve in situ regenerationBacteriaMicroorganism based processesAlcoholEnantiomer
The invention belongs to the technical field of gene engineering and particularly relates to carbonyl reductase AcCR and an encoding gene and application thereof. The amino acid sequence of the carbonyl reductase AcCR is shown as SEQ ID NO.1. The nucleotide sequence of the gene for encoding the carbonyl reductase AcCR is shown as SEQ ID NO.2. The carbonyl reductase AcCR comes from bacillus aceticus XZY003 and can catalyze 13 carbonyl compounds to generate corresponding chiral alcohol of single enantiomers. The carbonyl reductase AcCR and glucose dehydrogenase GDH are co-expressed in a prokaryotic expression system or a eukaryotic expression system, the biological reaction process of biological catalytic conversion is conducted through the carbonyl reductase, in-situ regeneration of coenzyme is achieved, production cost is greatly reduced, the optical purity of an obtained product is high, the reaction process is efficient, and conditions are moderate.
Owner:SOUTH CHINA UNIV OF TECH
Alcohol dehydrogenase mutant and application thereof to synthesis of diaryl chiral alcohol
InactiveCN108384765AHigh stereoselectivityIncrease vitalityBacteriaMicroorganism based processesFormate dehydrogenase HGlycol synthesis
The invention discloses an alcohol dehydrogenase mutant and application thereof to synthesis of diaryl chiral alcohol and belongs to the technical field of biological engineering. The alcohol dehydrogenase mutant disclosed by the invention has good catalytic activity and stereoselectivity, and a series of chiral diaryl alcohol with R- and S- configurations can be prepared through efficient catalysis. According to the alcohol dehydrogenase mutant, alcohol dehydrogenase is coupled with glucose dehydrogenase or formate dehydrogenase, and can be used for synthesizing various antihistamine drugs, i.e., a chiral diaryl alcohol intermediate. Compared with an existing report, a method for preparing the diaryl chiral alcohol through asymmetric catalysis of the alcohol dehydrogenase has the advantages of simplicity in operation, high substrate concentration, complete reaction and high product purity and has a very good industrial application prospect.
Owner:JIANGNAN UNIV
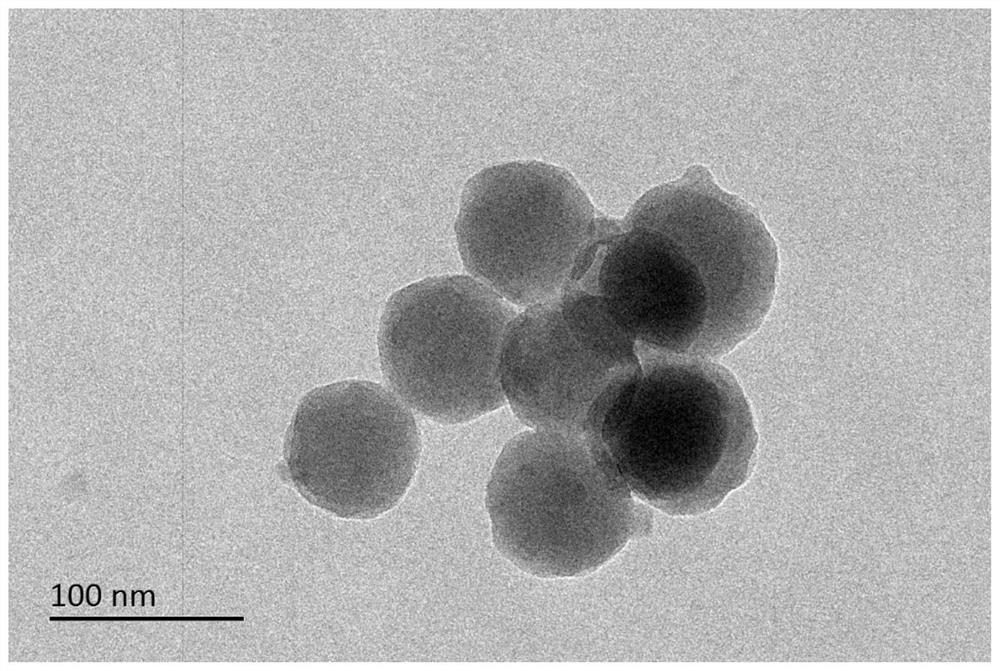
Protein and amorphous metal organic framework compound and preparation method thereof
ActiveCN111909924AHigh embedding rateImprove stabilityOxidoreductasesCarrier-bound/immobilised peptidesKetoneOxidative enzyme
The invention discloses a protein and amorphous metal organic framework compound and a preparation method thereof. The organic framework compound has a 2 nm-50 nm mesoporous structure. The preparationmethod comprises the following step of enabling a protein, zinc ions and an organic ligand to react in a solvent, wherein the organic ligand is a compound containing an imidazole group; the protein is one or a combination of several kinds of cytochrome C, cytochrome P450, horse radish peroxidase, alcohol dehydrogenase, lipase, acetylcholin esterase, laccase, a green fluorescent protein, glucose dehydrogenase, glucose oxidase, trypsin, bacillus subtilis protease, carbonic anhydrase, aldehyde ketone reductase, amylase, saccharase, superoxide dismutase, urease and catalase. The preparation method of the protein and amorphous metal organic framework compound provided by the invention is simple to operate and mild in condition, the obtained product is high in protein embedding rate and good inprotein stability, and the biologic al activity of the protein is reserved to a greater extent.
Owner:TSINGHUA UNIV
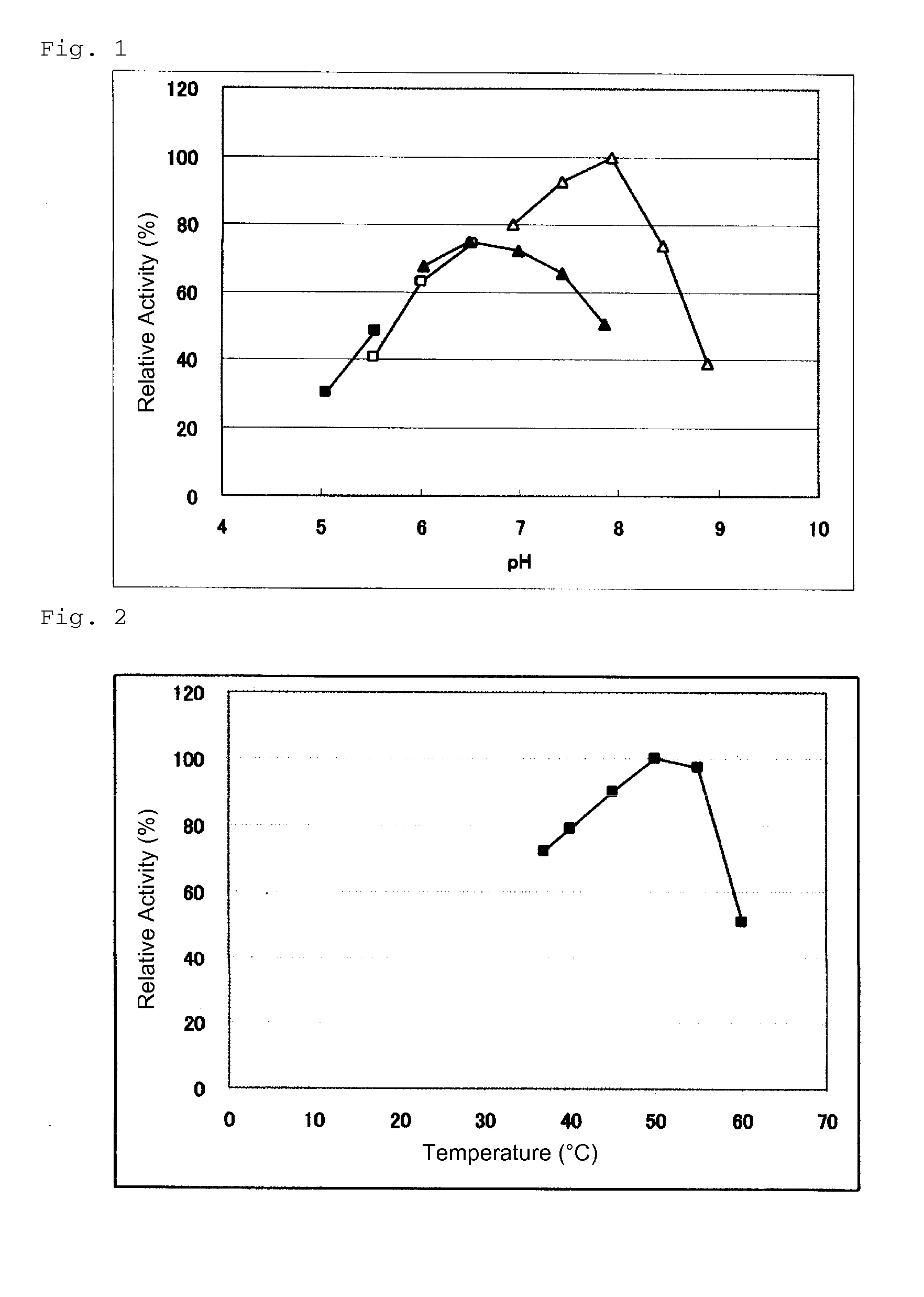
Novel glucose dehydrogenase
ActiveUS20150031059A1High affinityReduced responseSugar derivativesMicroorganismsElectrophoresesFlavolipin
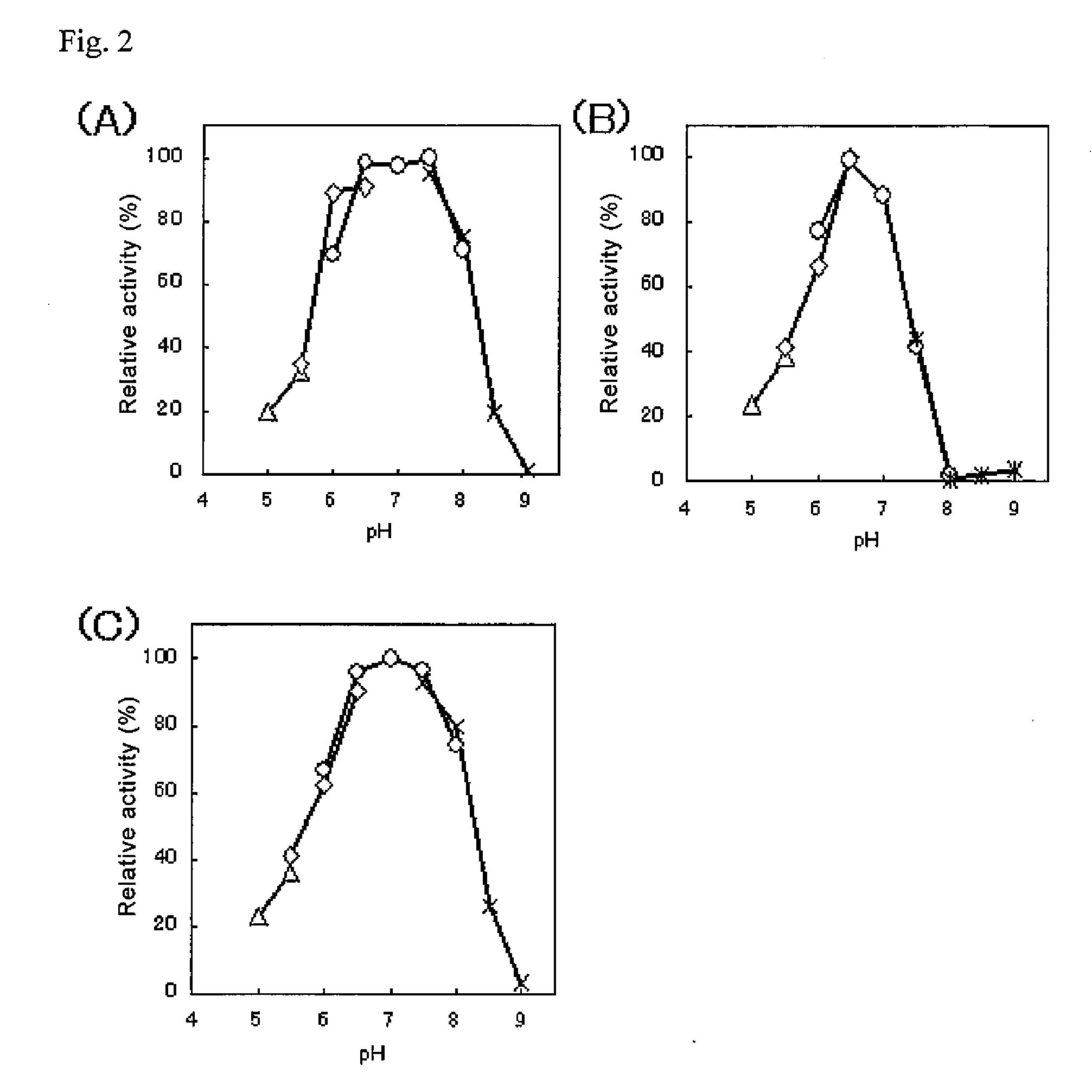
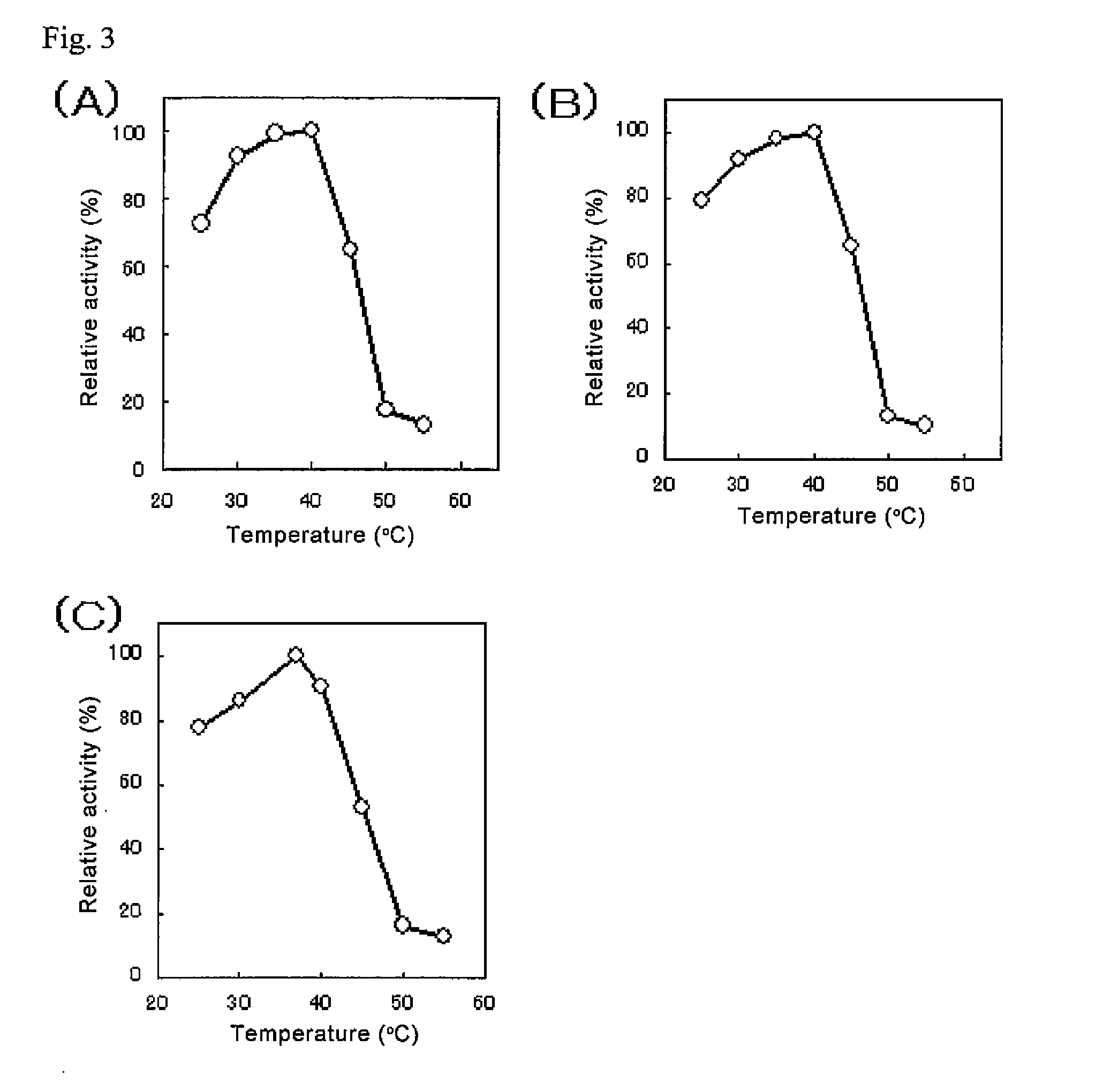
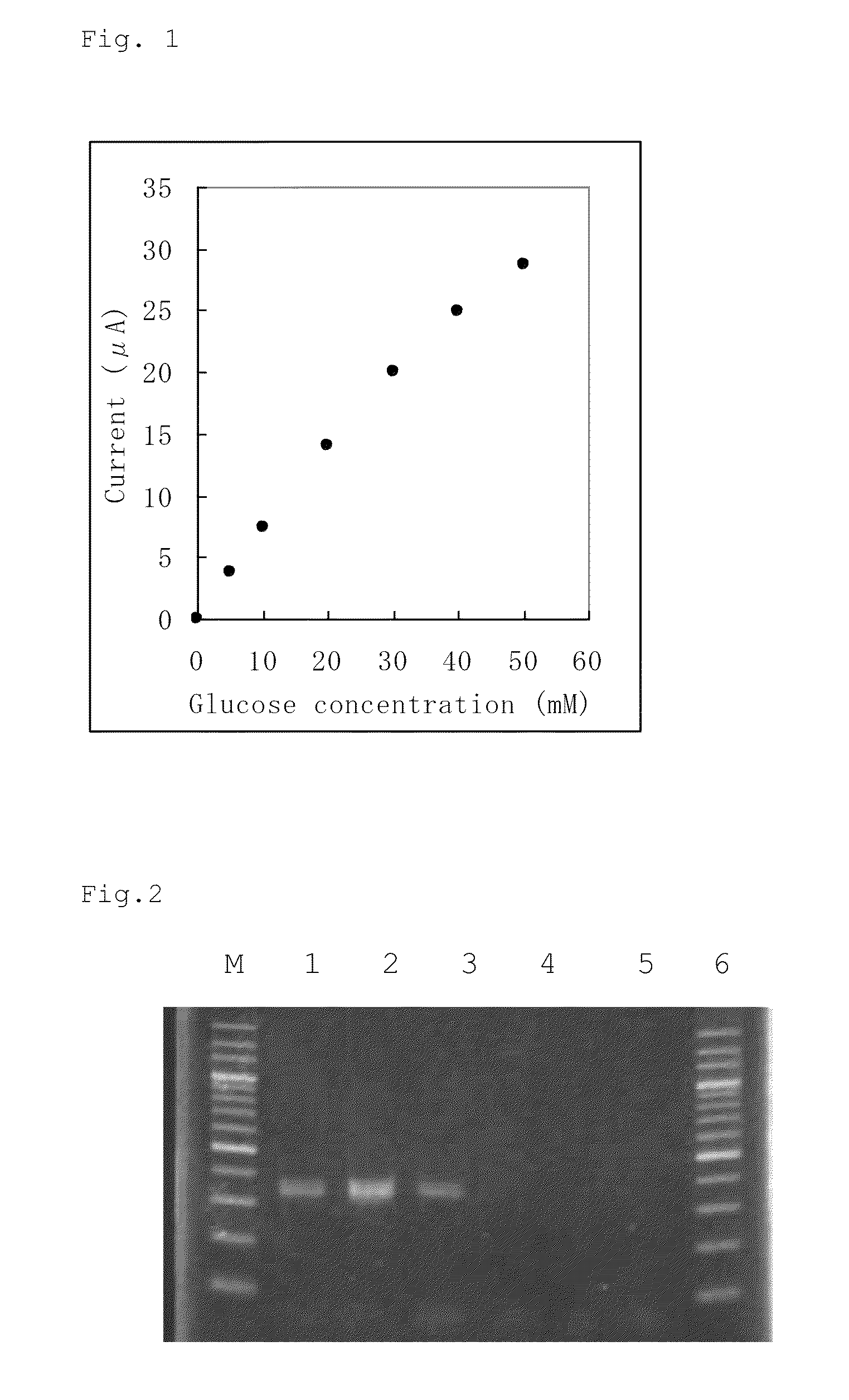
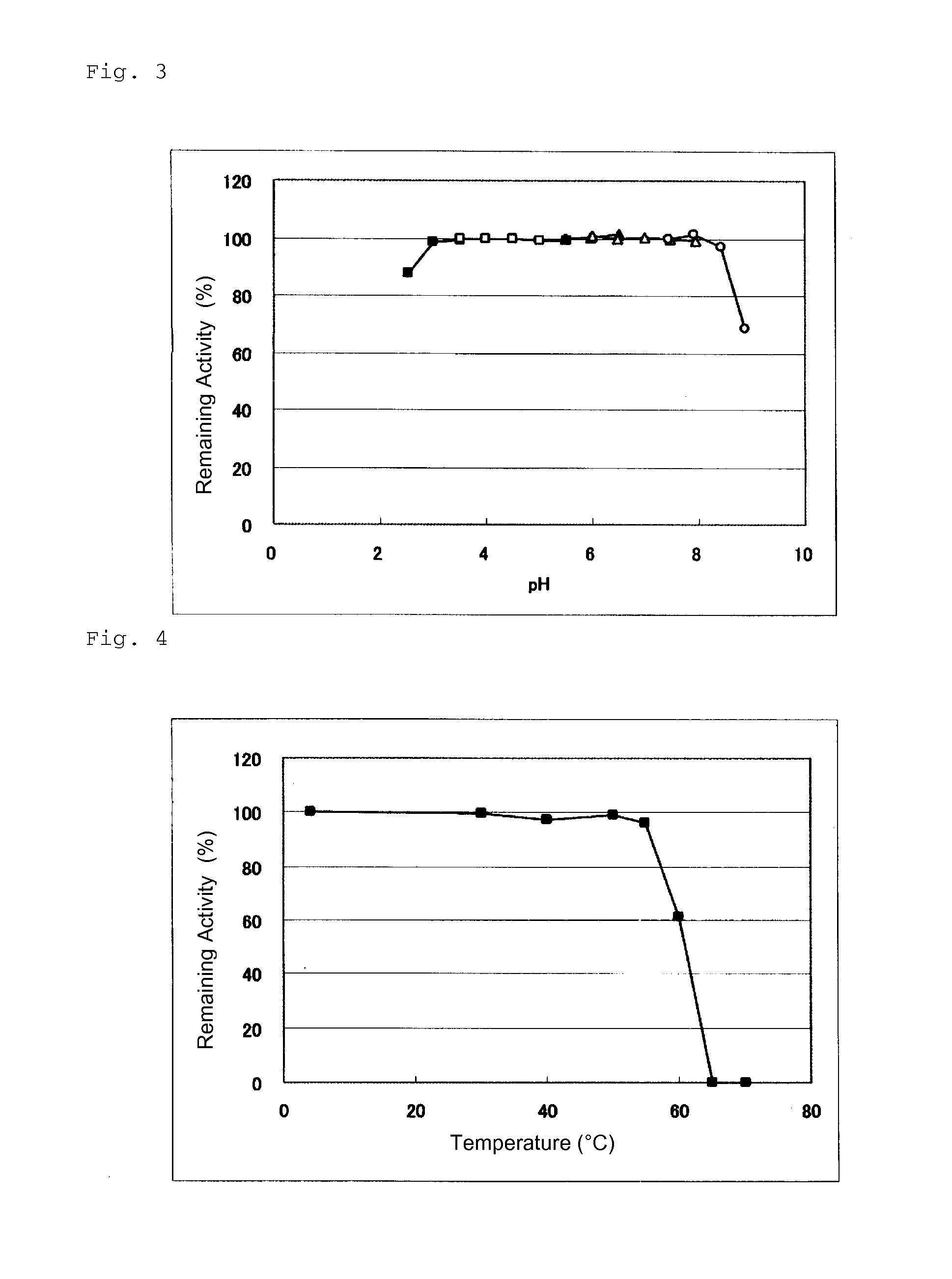
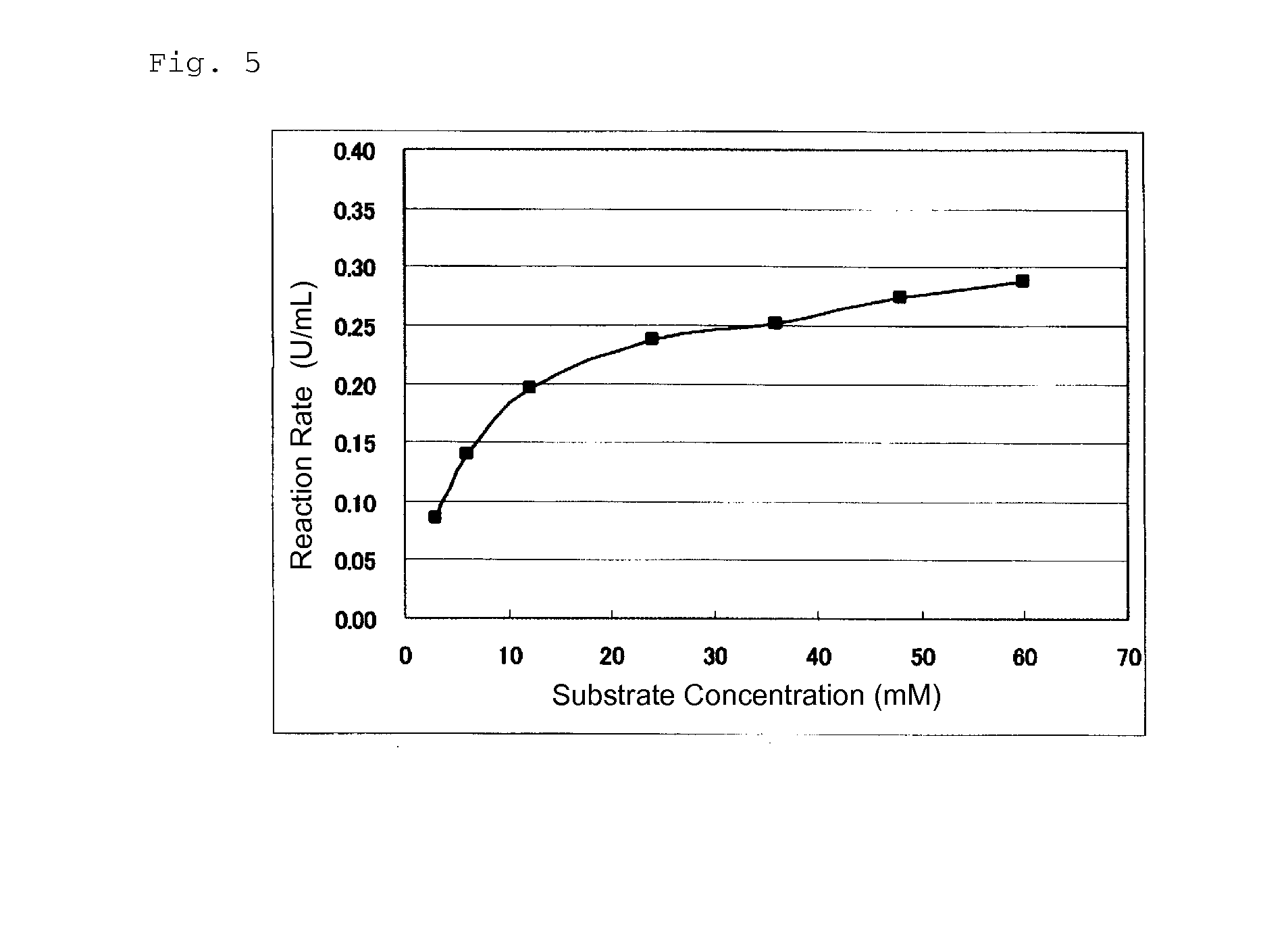
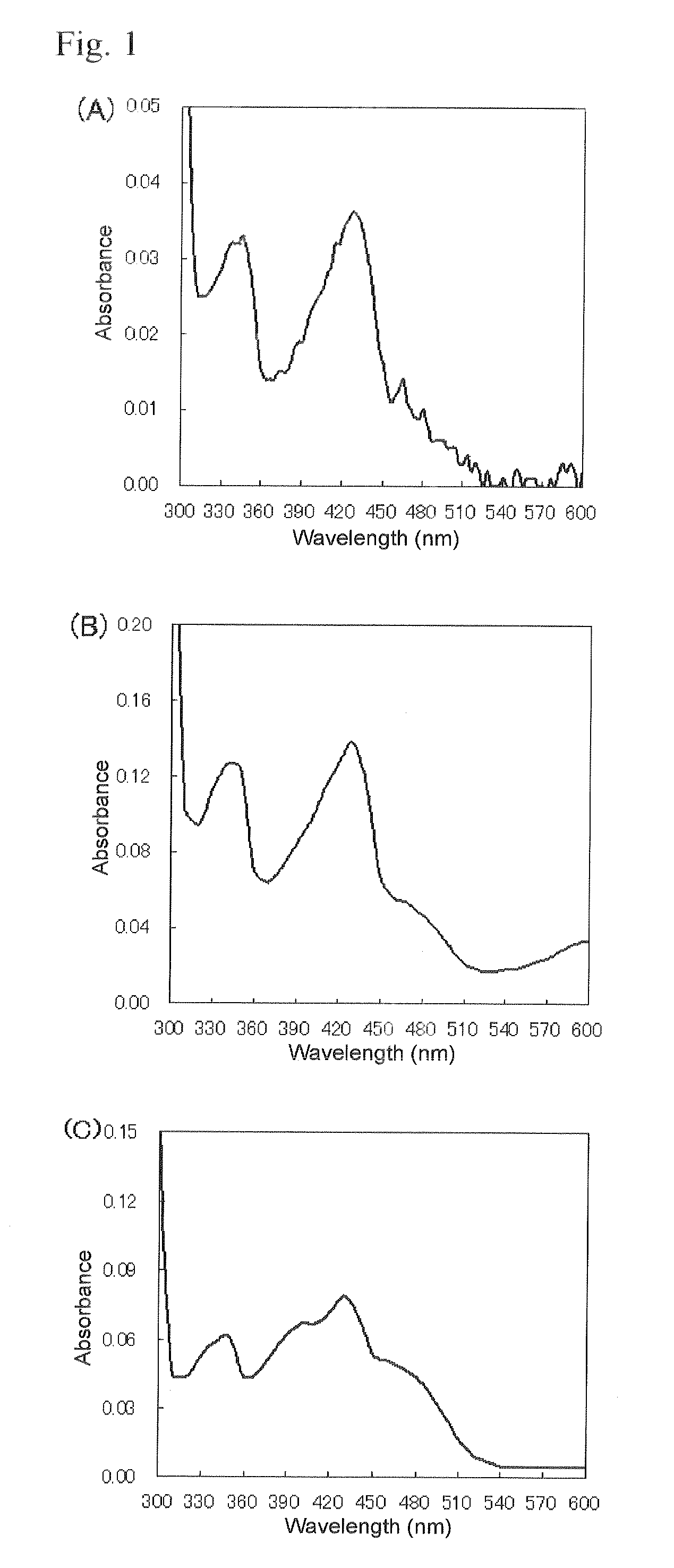
An object of the present invention is to provide a novel glucose dehydrogenase, a method for producing the glucose dehydrogenase, and applications of the glucose dehydrogenase. The flavin-binding glucose dehydrogenase of the invention has the following characteristics (1) and (4): (1) Molecular weight: the molecular weight of a polypeptide moiety in the enzyme is about 68 kDa as measured by SDS-polyacrylamide electrophoresis; (2) Km value: the Km value for D-glucose is about 15 mM or less; (3) Temperature stability: stable at a temperature of 55° C. or less; and (4) pH stability: stable at a pH range of 3.0 to 8.5.
Owner:TOYOBO CO LTD
Flavin-binding glucose dehydrogenases
ActiveUS8445246B2Accurate measurementLeveling precisionSugar derivativesBacteriaMicroorganismFlavolipin
A flavin-binding glucose dehydrogenase with a high substrate specificity for D-glucose. The flavin-binding glucose dehydrogenase which is derived from a microorganism belonging to the genus Mucor. The flavin-binding glucose dehydrogenase has a low reactivity for maltose, D-galactose and D-xylose compared to its reactivity for D-glucose, and therefore is relatively unaffected by these saccharide compounds. The flavin-binding glucose dehydrogenase is also relatively unaffected by dissolved oxygen, and allows accurate measurement of glucose amounts even in the presence of saccharide compounds other than glucose in samples.
Owner:KIKKOMAN CORP
Novel glucose dehydrogenase
ActiveUS20140287478A1High affinityReduced responseMicrobiological testing/measurementOxidoreductasesGlucose dehydrogenase activityDehydrogenase
An object of the present invention is to provide a novel glucose dehydrogenase, a method for producing the glucose dehydrogenase, and applications of the glucose dehydrogenase. An isolated flavin-binding glucose dehydrogenase comprising a polypeptide having an amino acid sequence with 80% or more identity to the amino acid sequence of SEQ ID NO: 1, and having glucose dehydrogenase activity is provided.
Owner:TOYOBO CO LTD
Glucose dehydrogenase mutant, preparation method thereof and application
ActiveCN106754777AImprove toleranceHigh catalytic activityBacteriaMicroorganism based processesEscherichia coliNucleotide
The invention discloses a glucose dehydrogenase mutant, a preparation method thereof and an application. A nucleotide sequence of a glucose dehydrogenase mutant gene is as shown in SEQ ID NO.1, and an amino acid sequence of the glucose dehydrogenase mutant is as shown in SEQ ID NO.2. The gene is led into Escherichia coli to obtain gene engineering bacteria with the gene, the gene engineering bacteria are cultured, and a fermentation process is optimized, so that recombinant glucose dehydrogenase is prepared. The glucose dehydrogenase mutant is good in catalytic activity, pH (potential of hydrogen) and heat stability and organic solvent tolerance and can be used for regeneration of coenzyme NADH and NADPH in oxidation-reduction reactions.
Owner:JIANGSU ALPHA PHARM CO LTD
Method for preparing (R)-phenylglycol from SD-AS sequence coupled (R)-carbonyl reductase and glucose dehydrogenase
InactiveCN104830744AHigh optical purityHigh yieldBacteriaMicroorganism based processesEscherichia coliRecombinant escherichia coli
The invention discloses a method for preparing (R)-phenylglycol from SD-AS sequence coupled (R)-carbonyl reductase and glucose dehydrogenase and belongs to the technical field of biological catalytic asymmetric transformation. The invention provides recombinant escherichia coli E. coli RIL / pET-R-SD-AS-G. The recombinant escherichia coli E. coli RIL / pET-R-SD-AS-G is preserved in the China center for type culture collection (CCTCC) and has a preservation number of CCTCC NO: M2015170. R-carbonyl reductase and glucose dehydrogenase are coupled by a coexpression way, and the coupled enzyme, a cultured recombinant strain and 2-hydroxyacetophenone as a substrate undergo a catalytic asymmetric transformation reaction under optimized biotransformation reaction conditions to produce (R)-phenylglycol with optical purity of 100% and a yield of 99.9%. The method utilizes a SD-AS sequence high efficiency coupling double-enzyme technology, solves the problem of limitation of chiral catalytic reaction coenzyme regeneration cycle and provides an effective approach for high efficiency bio-preparation of the (R)-phenylglycol.
Owner:JIANGNAN UNIV
Recombinant yeast for unsymmetrical conversion and preparation of (S)-4-chloro-3-hydroxybutanoate and construction method and use thereof
InactiveCN101392225AImprove conversion rateHigh optical activityFungiMicroorganism based processesBiotechnologyEnzyme Gene
The invention discloses recomposed yeast for asymmetrically transforming and preparing (S)-4-chlorine-3-hydroxy-ethylbutyrate, which is Saccharomyces cerevisiae for reducing enzyme gene and glucose dehydrogenase gene by the introduction of carbonyl. The invention further discloses a composition method of the recomposed yeast. The method for preparing (S)-4-chlorine-3-hydroxy-ethylbutyrate by using the recomposed yeast is that: 4-chloracetyl-ethyl-acetate is used as a substrate, glucose is used as an auxiliary substrate, and the recomposed yeast is used for the transformation reaction so as to prepare (S)-4-chlorine-3-hydroxy-ethylbutyrate. The recomposed yeast, without adding any auxiliary enzyme, can effectively catalyze 4-chloracetyl-ethyl-acetate into (S)-4-chlorine-3-hydroxy-ethylbutyrate, with optical purity e.e value larger than 98 percent and the transformation ratio to the substrate larger than 95 percent, thus reducing the production cost. Compared with other strains without the need to add expensive auxiliary enzyme, the recomposed yeast has high transformation ratio to the substrate and high optical activity.
Owner:NANJING UNIV OF TECH
Strategy for efficiently coproducing alpha-aminobutyric acid and gluconic acid
ActiveCN105255934AGood market demandThe conversion process is fast and efficientFermentationVector-based foreign material introductionEscherichia coliEnzyme Gene
The invention relates to a method for coproducing alpha-aminobutyric acid and gluconic acid by constructing recombinant escherichia coli by virtue of series connection of glucose dehydrogenase and L-amino acid dehydrogenase. According to the method, recombinant co-expression vectors are constructed by virtue of glucose dehydrogenase genes and L-amino acid dehydrogenase genes and are transferred into escherichia coli of genetically engineered bacteria; meanwhile, recombinant escherichia coli which expresses L-amino acid dehydrogenase is constructed; by virtue of efficiently co-expressing glucose dehydrogenase and L-amino acid dehydrogenase into escherichia coli, the circulation of cofactors in mycetome can be promoted, and by virtue of a cyclic regeneration system of the cofactors, high-added-value alpha-aminobutyric acid and gluconic acid can be coproduced from cheap substrates, namely L-amino acid and glucose without adding any exogenous cofactor; the transfer process is simple and rapid, and the cost is low; the yields of alpha-aminobutyric acid and gluconic acid produced in a 5L fermentation tank by virtue of the method can respectively reach 102.8g / L and 196.8g / L, and a practical and effective strategy is provided for industrial production.
Owner:ANHUI HUAHENG BIOTECH
Preparation method for dehydroepiandrosterone, and enzyme for preparation thereof
InactiveCN109312382AHigh yieldSimple processMicroorganismsMicroorganism based processesSodium ascorbate4-Androstenedione
A preparation method for dehydroepiandrosterone, comprising: in a protective atmosphere, adding potassium tert-butoxide to tert-butanol, stirring evenly, adding 4-androstenedione to obtain a mixture,and adding the mixture dropwise to a sodium ascorbate-containing acetic acid solution for a reaction to obtain 5-androstenedione; dissolving the 5-androstenedione in an organic solvent, adding a ketone reductase, a glucose dehydrogenase, glucose and a redox coenzyme to obtain a mixture, controlling the pH of the mixture to be 6.0-6.3, stirring and reacting for 1-6 hours at 22-26 DEG C to obtain areaction solution, and performing separation and purification on the reaction solution to obtain dehydroepiandrosterone, the ketone reductase and the glucose dehydrogenase being coexpressed by a microbial strain and added in the form of a crude enzyme solution. The synthesis process of the preparation method has few steps, simple operations, high yields and low costs, and may be widely applied toindustrial scale production. Also provided is an enzyme for preparation.
Owner:BONTAC BIO ENG SHENZHEN
Phosphinothricin dehydrogenase mutant, genetically engineered bacteria and one-pot multi-enzyme synchronous directed evolution method
ActiveCN111621482AImprove conversion rateIncreased space-time yieldBacteriaMicroorganism based processesArginineThreonine
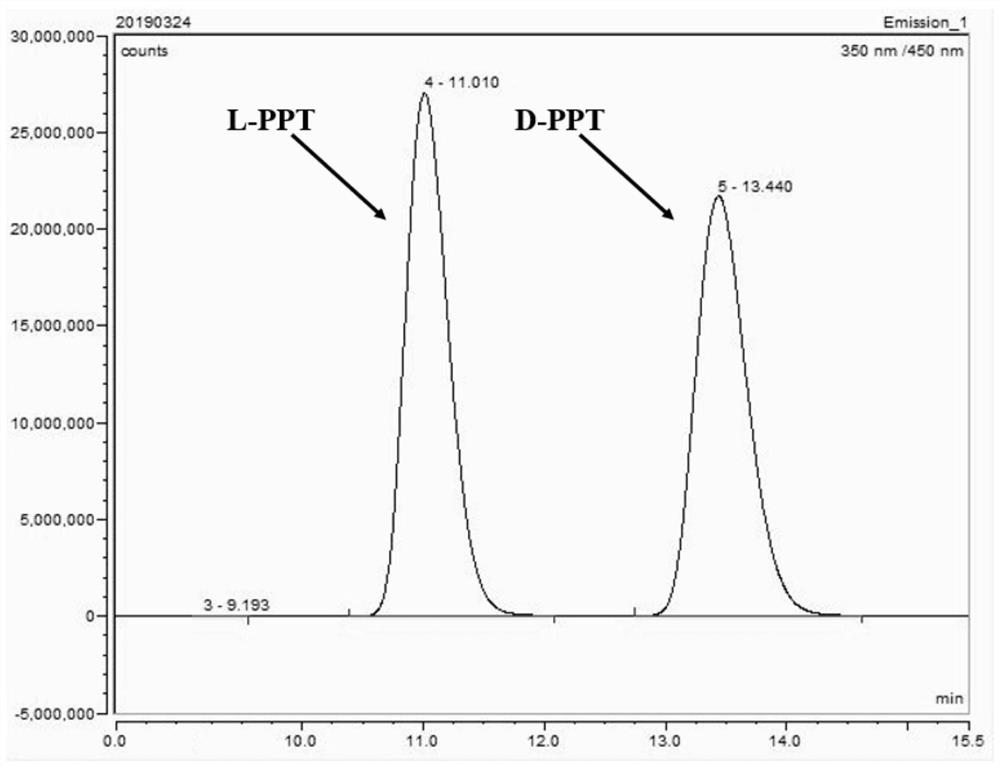
The invention discloses a phosphinothricin dehydrogenase mutant, genetic engineering bacteria and a one-pot multi-enzyme synchronous directed evolution method. The phosphinothricin dehydrogenase mutant is obtained by mutating the 164th amino acid from alanine to glycine, mutating the 205th arginine to lysine and mutating the 332nd threonine to alanine of phosphinothricin dehydrogenase derived fromPseudomonas fluorescens, and an amino acid sequence is as shown in SEQ ID No.1. The genetically engineered bacteria are obtained by introducing a gene of the phosphinothricin dehydrogenase mutant into a host cell. An encoding gene of glucose dehydrogenase or an encoding gene of formate dehydrogenase can also be introduced into the host cell to perform simultaneous directed evolution to overexpress the double genes. The one-pot multi-enzyme synchronous directed evolution method of the invention can screen out the genetically engineered bacteria with greatly improved activity. Compared with catalytic processes such as transaminase, the L-PPT preparation method of the invention has a relatively simple process, a high conversion rate of raw materials, a conversion rate of up to 100%, and highstereo-selectivity.
Owner:ZHEJIANG UNIV OF TECH
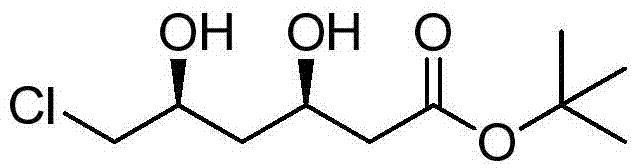
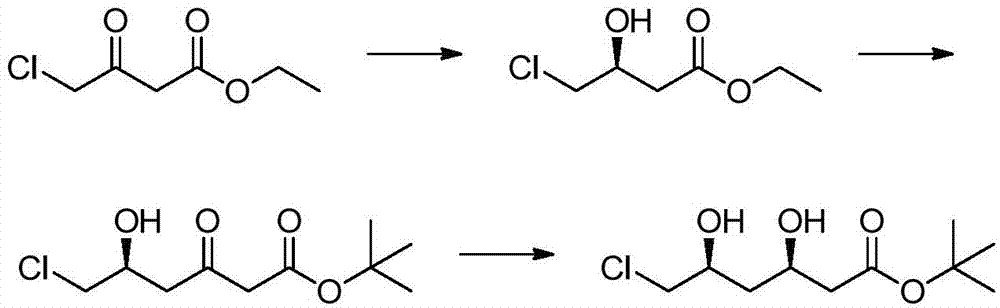
Preparation method of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate
The invention relates to a preparation method of tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate. The preparation method is characterized in that tert-butyl (S)-6-chloro-5-hydroxy-3-carbonylhexanoate is taken as a substrate and the substrate is subjected to reduction reaction in the presence of a biocatalyst, a coenzyme and coenzyme regeneration systems to generate tert-butyl (3R,5S)-6-chloro-3,5-dihydroxyhexanoate; the biocatalyst is ketoreductase; the coenzyme is NADP (nicotinamide adenine dinucleotide phosphate); the coenzyme regeneration systems are glucose and glucose dehydrogenase; the reduction reaction is carried out in a phosphate buffer solution at 25-30 DEG C in the presence of an enzyme protection reagent; the pH value of a reaction system is controlled to be 6.0-8.0 by adding a sodium carbonate solution. The preparation method has the effects of substantially increasing the concentration of the substrate and reducing the usage amount of the enzyme, so that the product has good production environment friendliness and the commercial value of industrialization is prominent.
Owner:ENZYMEWORKS
Mutant glucose dehydrogenase
A mutant glucose dehydrogenase having an amino acid sequence at least 80% identical to SEQ ID NO:3 and having glucose dehydrogenase activity, wherein amino acid residues corresponding to positions 326, 365 and 472 of said amino acid sequence are replaced with glutamine, tyrosine and tyrosine, respectively, and wherein said mutant glucose dehydrogenase shows an improved substrate specificity to glucose and a reduced reactivity to disaccharides.
Owner:ARKRAY INC
Transformant transfected with flavin adenine dinucleotide-binding glucose dehydrogenase gene and method for producing flavin adenine dinucleotide-binding glucose dehydrogenase using the same
InactiveUS20110053194A1Accurate measurementIncrease production capacityFungiMicrobiological testing/measurementNucleotideA-DNA
It is intended to highly efficiently produce a large amount of novel FAD-GDH capable of more accurately measuring a glucose level. Provided is a transformant in which a DNA encoding a flavin adenine dinucleotide-binding glucose dehydrogenase selected from the group consisting of (a) a DNA encoding the amino acid sequence of SEQ ID NO: 20; (b) a DNA consisting of the base sequence of SEQ ID NO: 19; (c) a DNA having a base sequence homologous to the base sequence of SEQ ID NO: 19 and encoding a protein having a flavin adenine dinucleotide-binding glucose dehydrogenase activity; (d) a DNA encoding the amino acid sequence of SEQ ID NO: 34; (e) a DNA consisting of the base sequence of SEQ ID NO: 33; and (f) a DNA having a base sequence homologous to the base sequence of SEQ ID NO: 33 and encoding a protein having a flavin adenine dinucleotide-binding glucose dehydrogenase activity has been introduced. Further, a method for producing a flavin adenine dinucleotide-binding glucose dehydrogenase using the transformant is provided.
Owner:AMANO ENZYME INC
L-amino acid-producing microorganism and a method for producing an l-amino acid
ActiveUS20110212496A1Efficient productionAbility to produceBacteriaOxidoreductasesMicroorganismPyrroloquinoline quinone
An L-amino acid is produced by culturing a bacterium belonging to the family Enterobacteriaceae, which has an L-amino acid-producing ability and inherently has a native activity of a glucose dehydrogenase that uses pyrroloquinoline quinone as a coenzyme, but has been modified so that the activity of the glucose dehydrogenase is reduced, in a medium, and collecting the L-amino acid from the medium.
Owner:AJINOMOTO CO INC
Glucose dehydrogenase
InactiveUS20040265828A1Improve purification effectEasy to purifyBioreactor/fermenter combinationsFungiSide chainBinding site
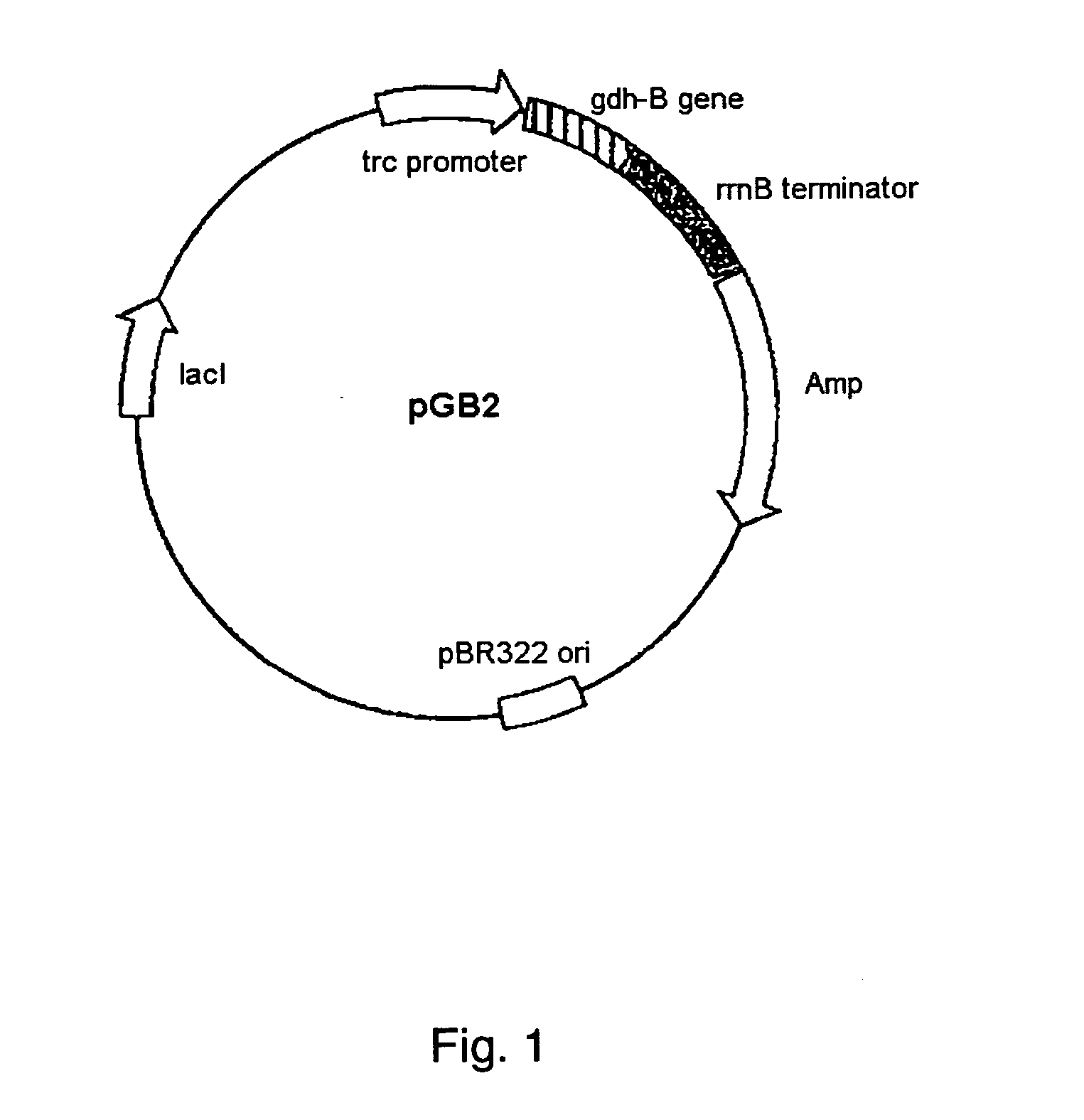
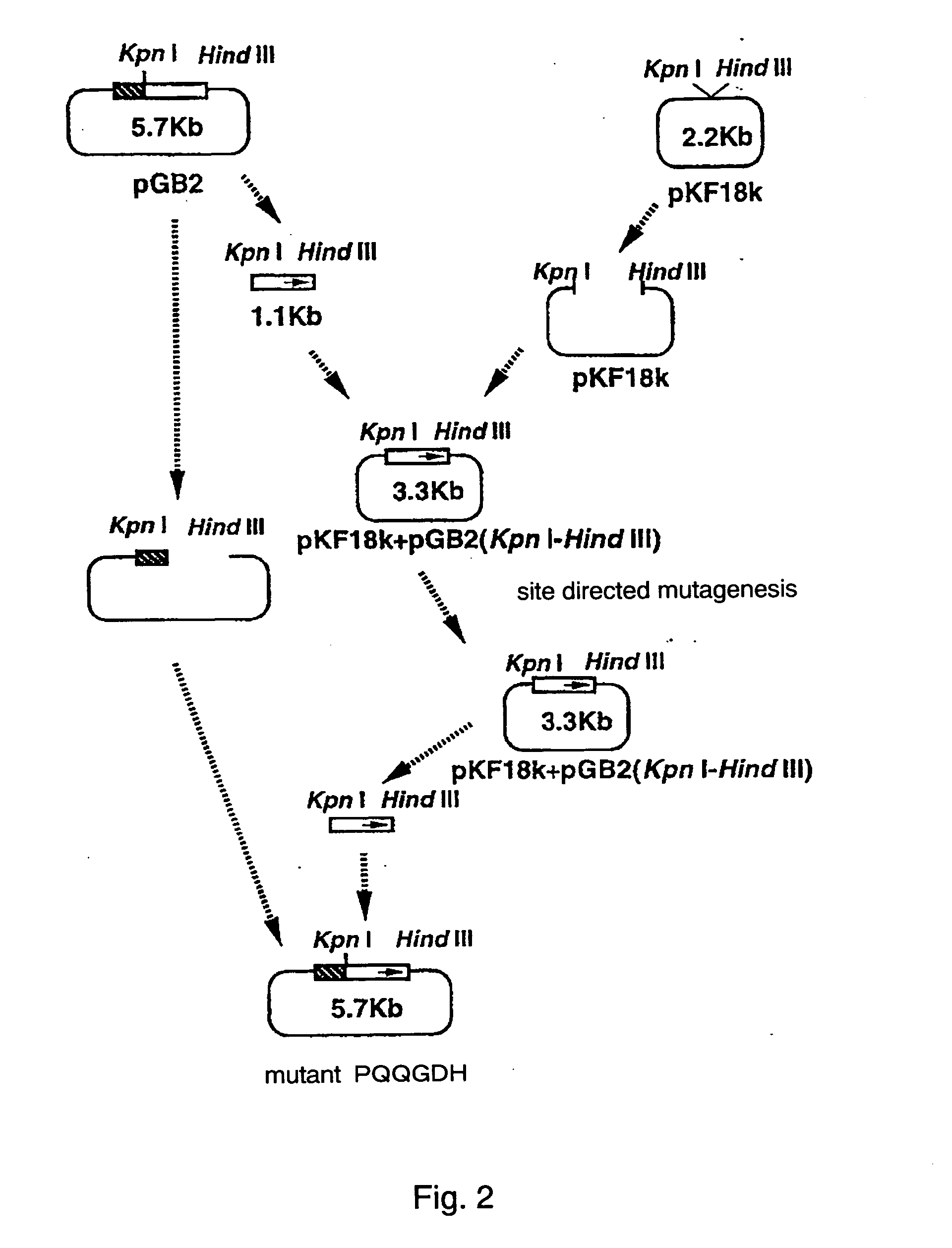
A modified glucose dehydrogenase is disclosed which comprises a water-soluble glucose dehydrogenase having a pyrroloquinoline quinone as a coenzyme. At least one amino acid residue present on the surface of the water-soluble glucose dehydrogenase is replaced with arginine. The side chain of the amino acid residue is exposed at the surface of the molecule and is not expected to substantially interact with other residues. The amino acid residue is present in a region that is probably not an enzyme active site or a substrate binding site. Preferably the amino acid residue is selected from the group consisting of glutamine, asparagine, and threonine. This modified enzyme can be prepared by recombinant processes and recovered efficiently.
Owner:ULTIZYME INT LTD
Pyrroloquinoline quinone-dependent glucose dehydrogenase
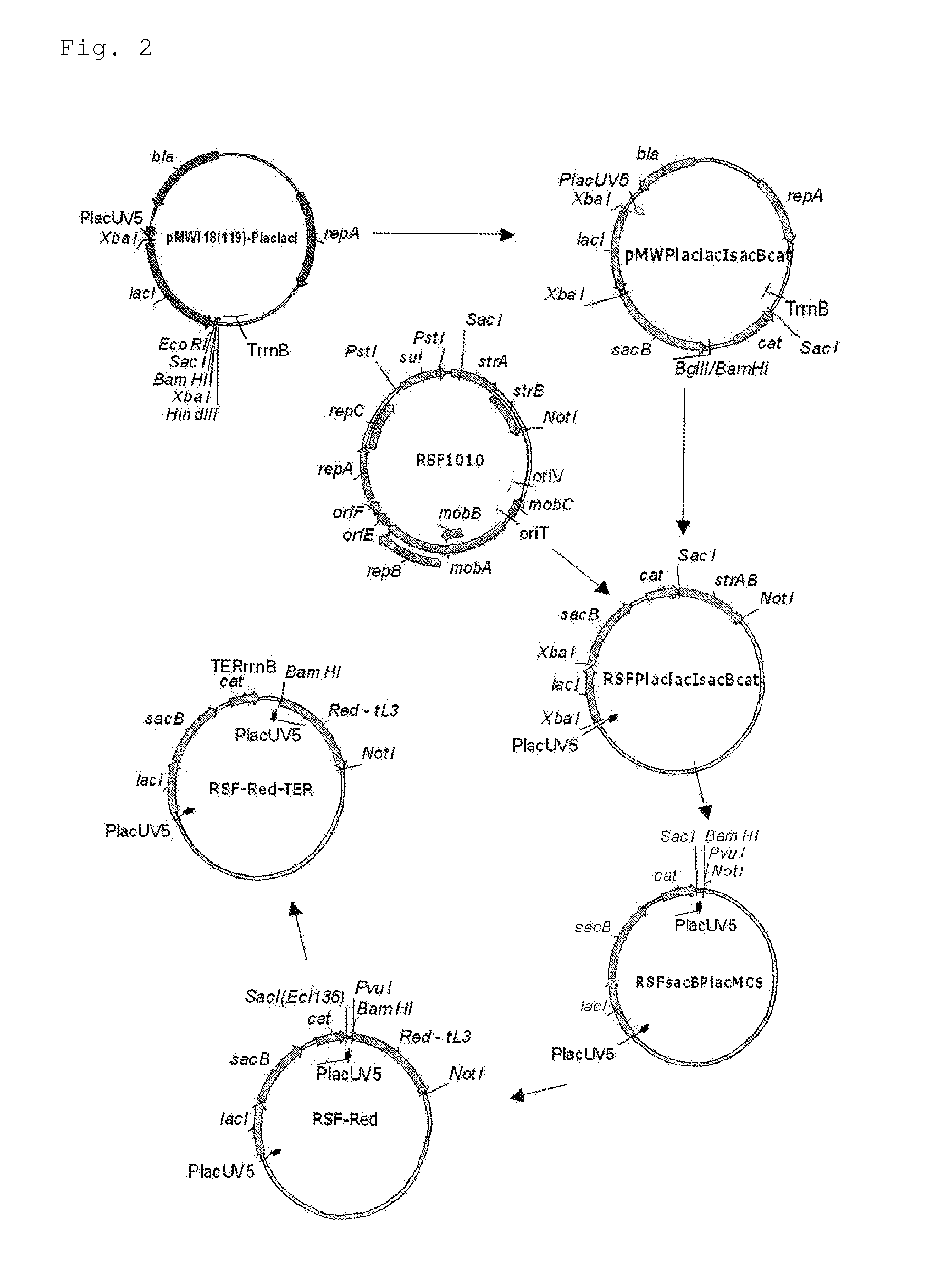
InactiveUS7037698B2Reduced responseIncreased substrate specificityFungiSugar derivativesAmino acid compositionAcyl CoA dehydrogenase
A modified pyrroloquinoline quinone-dependent glucose dehydrogenase having a low reactivity with respect to maltose, galactose, etc. is provided. A modified pyrroloquinoline quinone-dependent glucose dehydrogenase including an amino acid sequence in which one or more amino acids in a region corresponding to a first region consisting of amino acids at positions 326 to 354 in pyrroloquinoline quinone-dependent glucose dehydrogenase derived from Acinetobacter calcoaceticus are substituted as compared with an amino acid sequence of the corresponding wild-type enzyme.
Owner:AMANO ENZYME INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com